What is Microscopy Imaging?
What Silicon Wafers are Used for Microscopy Imaging?
Researchers of organic nano electronics have used the following wafer spec for their microscopy imaging. Please let us know how we can help you!
Si Item #857 - buy as few as one wafer
150mm P/B <100> 0-10 ohm-cm 620um SSP Test Grade
These wafers work perfectly for microscopy imaging
Start researching today!
Key Microscopy Imaging
- microscopy imaging
- confocal microscopy
- microscopes resolution
- field microscopy
- microscopy allow
- scanning microscope
- multiphoton microscopy
- capacitance microscopy
- microscope images
- light microscopy
- panoramic microscope
- sheet microscopy
- photon microscope
- force microscopy
- electron microscopy
What is Microscopy Imaging?
What is Microscopy Imaging? What do these images look like? Let's find out. What are the benefits of microscopy? Here are some ways you can use it. It can help you understand more about the world we live in. It can also be used for research. Read on to learn more! Here are some of the most important advantages of microscopy imaging. Here are some examples. And, don't forget to check out our other articles on microscopy and its many applications.
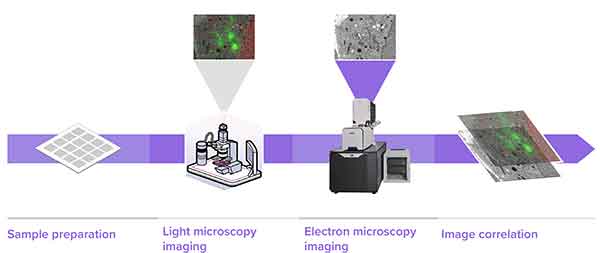
1. Sample Preparation, 2. Light microscopy imaging, 3. Electron microscopy imaging, 4. image correlation.
The most basic of microscopy imaging techniques consists of a light source and an enlargement lens. These devices allow researchers and scientists to see fine details in cells and biological processes. Because these images are created by using light, the microscope is a powerful tool. The microscopy imaging device determines how much detail you can see on a particular specimen, the resolution of relevant structures, and the dynamics of the whole process. But, the vast array of different imaging devices can make choosing the right microscope difficult. This article will provide a basic overview of the different types of microscopy and how to choose the right one.
Digital images are composed of brightness transitions that cycle from one level of light to another. The cycle rate between these transitions is called the spatial frequency. As a result, the CTF of a system will be lower than the average of its components. Small details with low contrast will appear less prominent as they pass through the system. As a result, the lowest CTFs are found in the CCD and objective. Digital to analog conversion (D2A) is a common method for increasing contrast.
Advantages and Disadvantages of Microscopy Imaging
The various methods of microscopy imaging are as varied as the instruments used. Light optical microscopy, fluorescence light microscopy, confocal laser scanning microscopy, and near-field optical scanning microscopy are examples of these methods. Regardless of the method used, the objective is the same: to capture information about a sample in high resolution. The image quality degrades rapidly the deeper the sample is buried in scattering tissue.
Light optical microscopy
A light optical microscope uses visible light to magnify samples. Other imaging techniques use X-rays,  electrons, or atomic forces to achieve higher resolutions. Both types of microscopes are equally effective for obtaining clear pictures of microscopically small objects. In this article, we will discuss two types of light microscopes used for microscopy imaging. A light microscope uses visible light to magnify samples and is commonly used for student projects.
electrons, or atomic forces to achieve higher resolutions. Both types of microscopes are equally effective for obtaining clear pictures of microscopically small objects. In this article, we will discuss two types of light microscopes used for microscopy imaging. A light microscope uses visible light to magnify samples and is commonly used for student projects.
Unlike conventional microscopes, light optical microscopes are not perfect. The light passing through the objective lens produces diffraction spots in the image plane. In many cases, two diffraction spots are so similar that they cannot be distinguished. Increasing magnification power does not enhance spatial resolution. The resolution of light-optical microscopes depends on the light source used. If an object is transparent, light optical microscopy is most suitable.
An example of an optical microscope is a photoresist on silicon. The photoresist is 1 mm thick and covers the entire visible area of the field of view. This allows trenches to be imaged using a microscope. The bottom focus image demonstrates that the trenches are not entirely clear of photoresist. Cloud plots confirm the hypotheses, revealing that the base of the rightmost trench has weaker reflected signal intensity.
The light illuminators used for light optical microscopy are usually able to switch from transmitted light to reflected light. A modern reflected light microscope may include a partially reflective plane glass surface for brightfield illumination and a fully silvered mirror with a centrally located clear aperture for darkfield illumination. Depending on the light source used, a light optical microscope can have four or six objective lenses. A microscope's stage is mechanically controlled, allowing the specimen to be moved in both x and y-directions. Usually, an optical microscope has a built-in light source for illumination, such as a tungsten-halogen bulb.
Fluorescence light microscopy
In addition to transmitting light, fluorescent microscopy uses reflected light to examine samples. The green fluorescent protein (GFP) is excited by blue light and emits green light. The fluorescent light passes through a pinhole and is detected using an imaging device. In the microscope, this light is separated from the surrounding light radiation by a filter. The fluorescent light is then converted into images by a detector.
To begin fluorescence microscopy, the microscope should be equipped with a xenon or mercury light source. If using a mercury light source, you will need to allow about 15 minutes to achieve constant illumination. Next, position the sample on the stage. Select the lowest-powered objective and adjust the stage and fine-focus knobs. Then, turn off unnecessary room lights. Finally, insert the appropriate filter cube, open the shutter, and observe the samples.
The objective used for fluorescence light microscopy should be equipped with a correction color to compensate for temperature and coverslip variations. Similarly, high-numerical-aperture oil immersion objectives can be used for imaging fixed specimens. But these objectives should be carefully selected, as autofluorescence tends to obscure the details of the specimen and produce a high background. However, it is worth noting that oil immersion objectives require higher-magnification filters to be effective.
Fluorescence microscopy is the preferred method for studying intracellular macromolecule distributions. The increased resolution of the fluorescent images increases their usefulness and reliability. Common questions to ask include: Does the distribution of the molecules indicate randomness, order, or randomness? Are the differences between molecules in the same cell correlated? You can see the correlation between different molecules by using fluorescence microscopy.
Confocal laser scanning microscopy
The term "confocal" is derived from the fact that a microscope uses multiple mirrors to focus the laser beam while allowing the sample to be observed through a tiny pinhole. This technique is not suitable for live imaging, but can create highly representative images of fixed specimens. Although the method is complex and complicated, it is the gold standard for microscopic imaging. The following are some of the advantages and disadvantages of confocal laser scanning microscopy.
One of the most important uses of confocal laser scanning microscopy is for the study of biofilms. The Borlee lab has extensively applied CLSM to study biofilms in flow-cell reactors. The technique provides a comprehensive view of the composition and distribution of biofilm components. Antibody-conjugated dyes are used to identify individual components of biofilms. A variety of imaging parameters are available, including spatial information, cell distribution, matrix composition, and morphology.
A crucial benefit of confocal laser scanning microscopy is its high resolution and contrast. The variable aperture of the microscope allows for the focus of fluorescence without the stray light that occurs outside the focus plane. Additionally, the variable aperture helps remove out-of-focus details. The images acquired by this microscope are often visualized using computer software. The quality of confocal images depends on the laser type, pinhole aperture size, and the PMT gain.
The wavelength of light used and the numerical aperture of the microscope objective determine the thickness of the focal plane. The objective lens and specimen also affect the thickness of the focal plane. These two factors make confocal microscopes useful for surface profiling and 3D imaging. However, these techniques have some disadvantages as well. Therefore, it is important to consider all the possible consequences before investing in a confocal microscope.
Near-field optical scanning microscopy
Near-field optical scanning microscopy, or NFOSM, has the advantage of breaking the far-field resolution limit through its use of evanescent waves. This technique enables scientists to view tiny structures at an incredibly high resolution. Here are three reasons NFOSM may be better than conventional imaging:
Firstly, it is more accurate. Unlike optical microscopy, NFOSM can resolve smaller objects, such as single cells. The light from the object can be identified and separated from scattered light from objects closer to the microscope. This method allows researchers to examine both small and large objects without interfering with the sample's internal structure. The resolution of NFOSM is typically in the range of one nm.
The resolution of conventional light microscopy is limited to 200 to 300 nanometers. New technologies have greatly improved this sensitivity and resolution. Researchers are now able to see individual atoms with incredible detail. Near-field optical scanning microscopy has the potential to break these resolution barriers. Near-field microscopes are also faster than conventional light microscopy. In addition, these techniques have improved contrast-enhancing mechanisms.
As light microscopy is a powerful analytical tool, its resolution must be continuously improved. However, conventional far-field light microscopy has a diffraction limit of 250 nm. By contrast, near-field optical scanning microscopy (NFOSM) was the first technique to break this limit and has the ability to achieve resolutions of l/60 or less. This technique is now used extensively in microscopy and is also sometimes referred to as SNOM or optical stethoscopy.
One of the main benefits of this microscope is its ultra-high resolution. Near-field scanning optical microscopes greatly expand the analytical toolbox of a microscopist. Resources for government and university laboratories include NFOSM as a reference instrument. In addition to detailed instructions, it is possible to view images created with this microscope in near-field. For example, Glenn Fried has provided schematics and a power point download for this technique.
Structured illumination microscopy
A method called superresolution structured illumination microscopy (SuperSIM) has been developed to improve the resolution of imaging samples using two different fluorescent dyes. The technique uses a wide field camera with a high numerical aperture and a scrambled laser source to separate the light into multiple diffracted orders. SuperSIM can image a sample at resolutions of 50 nm and has the potential to improve microscopy imaging by enabling scientists to image cells and proteins at a nanoscale scale.
Depending on the application, the method can be used in reflected or transmitted light microscopy. The fundamental principle behind Structured Illumination is that a high-frequency illumination pattern can render high-resolution information visible. The intensity of the moire fringes is proportional to the spatial frequency of the pattern. Furthermore, nonlinearity introduces high-frequency harmonics into the effective pattern, thereby increasing its resolution-enhancement power.
In contrast to conventional wide-field fluorescence microscopy, SR-SIM can reveal structures that are often masked by other, more complex structure. The technique also enables multicolor imaging by using multiple lasers with different excitation wavelengths. However, the spatial resolution is not nearly as good as that of other super-resolution microscopy techniques. And SIM is not always regarded as a super-resolution method because it produces artifacts during image reconstruction.
Super-resolution fluorescence microscopy offers the potential to follow dynamic nanoscale interactions in living cells. However, the underlying process requires intense light to follow. The intense light can perturb the physiology of the cells. Structured illumination microscopy, on the other hand, allows scientists to image live cells using orders of magnitude less light than conventional optical microscopy. However, SIM resolution is limited to two times that of conventional optical microscopes.
Video: Microscopy Techniques
