Silicon Oxide Wafers for Research & Production
Typical Silicon Oxide Wafer Quote
A researcher from a small company requested a quote for the following:
25pcs,150mm N/Ph (1-0-0) 1-10 ohm-cm,675±25μm 1st FILM: 10,000ű2% SILICON OXIDE ON BOTH SIDES 2nd FILM: 8,000ű3% LOW STRESS LPCVD SILICON NITRIDE ON BOTH SIDES REFRACTIVE INDEX: 2.30 ± .07 @ 632.8nm FILM STRESS: <100 MPa ROUGHNESS:<1nm
Reference #270575 for our quote.
Get Your Quote FAST!
What is Silicon Oxide?
Silicon oxide, also known as silica or SiO2, is a chemical compound composed of silicon and oxygen. It is the most abundant mineral in the Earth's crust and is a major component of sand, quartz, and many other minerals. Silicon oxide is a widely used material in a variety of applications, including glass, ceramics, and building materials. It is also used in the semiconductor industry to create the insulating layer on silicon wafers in microelectronic devices. Silicon oxide is highly stable and resistant to heat, making it an ideal material for these applications.
What Are the Functions of Silicon Oxide in the IC Fabrication Process?
During the IC fabrication process, silicon oxide functions in different ways. In this 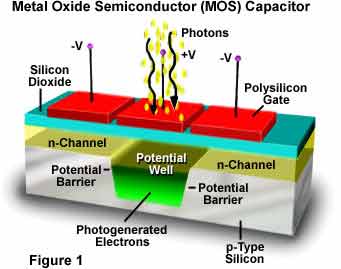 article, we will discuss some of these functions, such as thermal and vapour phase oxidation. These functions are essential in the IC fabrication process.
article, we will discuss some of these functions, such as thermal and vapour phase oxidation. These functions are essential in the IC fabrication process.
Vapour phase oxidation
During fabrication of an integrated circuit, silicon oxidation occurs many times. The process is carried out with a combination of oxygen and water. This method produces a silicon dioxide layer that thermally wraps the metal surface.
The oxidation process is characterized by an accelerated rate. The growth rate of the oxide is dependent on the concentration of the oxidant at the oxidized surface. The partial pressure of the oxidant in the surrounding gas is proportional to the rate of diffusion of the oxidant through the oxide. The growth rate is also dependent on the size and density of the initial oxide layer.
Oxidation can be divided into two stages, thermal oxidation and wet oxidation. Both processes are performed at high temperatures. The oxidant gas can be either pre-combusted hydrogen and oxygen or steam. The oxidant is introduced into the system after the equilibration stage has been completed. The temperature of the system can range from 700 to 1200 degC. The temperature profiles in the furnace must be accurately controlled to ensure uniformity.
The silicon oxidation process can be further subdivided into wet oxidation and dry oxidation. In wet oxidation, the substrate is soaked in a mixture of water and oxygen. The growth rate of the oxide is substantially higher than that of the dry oxidation process. This process can be used to produce thick oxide layers.
The wet oxidation process is also faster than the dry oxidation process. However, the impurity content in the wet oxidation process is significantly higher. Hence, the oxidation process is not recommended for thin oxide layers.
The growth rate of the oxide is dependent on a number of factors, including the chemical reaction rate, the concentration of the oxidant in the solid, and the velocity of the oxidant through the oxide layer. The temperature of the oxidation can be changed by 20 degrees Celsius. The oxidation rate is also affected by the amount of stress at the edge of the oxidizing area.
The growth rate is reduced by increasing the pressure of the oxidant. The pressure can be up to 20 atmospheres.
Thermal oxidation
During the manufacture of integrated circuits (ICs), thermal oxidation of silicon oxide is one of the most important steps. It is a process of chemically reacting silicon with oxygen in the ambient atmosphere to form silicon dioxide. The growth of silicon dioxide is controlled by a number of factors. These include the pressure, temperature, and time of oxidation.
The oxidation process is fast and produces only minimal defects. However, it is important to study the properties of the growing oxide so that the device can be produced reliably. In addition, the success of IC manufacturers depends on the ability to produce a uniform oxide film on the silicon surface.
The oxidation process occurs at low temperatures and has a rapid rate. The rate of oxidation can be increased by increasing the concentration of the oxidant or by increasing the pressure of the oxidant.
The oxidant can be pure oxygen or a mixture of hydrogen and oxygen. The oxidant gas is usually introduced after the system has equilibrated at the desired temperature. The flow of oxidant molecules causes the growth of the oxide and it is important to control the flow of the oxidant so that the dopants do not displace each other.
The chemical reaction between silicon and oxygen is limited by the number of silicon atoms available. It is possible to grow a selective oxide without trenching the silicon. The growth rate is also limited by the diffusion of oxygen through silicon dioxide. The desired oxide fills the vacancies in the bonds on the silicon surface. This provides a protective coating and conditions appropriate for lithography.
The oxidation process can be classified into two main sub-steps, wet oxidation and dry oxidation. The former involves the use of vapor from the wafer to oxidize the silicon, while the latter involves the use of molecular oxygen. The water vapor is more effective in forming thick oxides. This method is faster than the dry method.
The oxidation process can also be separated into the Linear Stage and the Parabolic Stage. In the Linear Stage, the growth rate is limited by the number of silicon atoms. In the Parabolic Stage, the growth rate is further increased by adding P-type dopants.
CVD
During the IC fabrication process, silicon is oxidized to form a thin oxide layer. The thickness of the oxide layer is dependent on the oxidation temperature and pressure. These processes can be done in a number of ways. These methods include thermal, wet and dry oxidation. A common method is the dry oxidation, which uses an oxygen gas as an oxidizing agent.
During the oxidation process, oxygen and silicon react to form silicon dioxide. This amorphous material is then deposited on the silicon substrate. The growth rate of the oxide is dependent on the pressure, temperature and the reaction time. The uniformity of the composition layer is also dependent on these factors.
The chemistry of the oxidation process begins at room temperature. The reaction is halted when the silicon oxide layer is thin. This occurs because the excess silicon atoms coalesce on the nucleation site. The bond strength between the Si-O interface is high. This bond strength can be used to passivate the silicon surface. It allows the silicon surface to rid itself of "dangling" bonds.
A second oxidation process involves the treching of the silicon wafer before it is oxidized. This process is often performed in LPCVD systems.
Another technique, called the pyrogenic process, involves the combination of hydrogen and oxygen gases. This process produces water vapor of a high purity. This process is useful in situations where the purity of the water is not ideal.
The thickness of the oxide layer can be determined experimentally or by the use of measuring equipment. To achieve a uniform thickness, the process must be carried out at a particular temperature and pressure. This is crucial for avoiding inversion of a lightly doped silicon substrate.
The oxidation temperature must be high enough to produce a viscous flow. It must also be high enough to relieve the stress that is present in the oxides. A low oxidation temperature reduces the growth rate of the oxide. This is particularly important in multi-step oxidation processes.
The growth rate of the oxide is affected by the ion source, the oxidation temperature and the kinetic energy of the ions. The amount of impurities and contamination is also affected. This can affect the electrical characteristics of the device.
Interpoly capacitors
During the fabrication of ICs, the use of silicon oxide is common. This material provides excellent insulators for microelectronic devices. It is also used as a mask against many impurities. It has the ability to form excellent MOS capacitors. In addition, it is suitable for device isolation via selective oxidation of silicon. It also has good barrier properties to water and sodium. Its dielectric constant is 3.9. It is one of the most widely used materials for capacitors.
When used in capacitors, the SiO2 layer is a very thin insulator. This allows the active and passive elements to be stacked on the same substrate. The thickness of the silicon dioxide film can range from a few to a few hundred angstroms. It is highly tolerant to thermal shock, and has an extremely high breakdown voltage. It can be introduced into the silicon substrate as a gas, steam, or high purity gas.
In the conventional technique of forming capacitors, the thin SiO2 film is deposited on the silicon substrate. A second polysilicon layer is deposited on the top of the silicon dioxide layer. This process is called a thin ONO dielectric composite. The layer has a thin film thickness and has reduced defect density. In addition, this layer is able to form a high capacitance interpoly dielectric structure.
An exemplary method of forming an ONO dielectric composite is described below. This method consists of deposition techniques, including wet oxidation and dry oxidation. The wet oxidation process has a better electrical behavior, and the growth rate is faster. It is characterized by the use of a small amount of co-reactant oxygen. This method can be used to replace ONO films in flash cells. It also has the potential to be used as gate oxides in more advanced devices.
The present invention also provides a method for forming a silicon nitride film in an ONO dielectric composite. This method combines a silicon nitride layer with a thin silicon dioxide film to provide a high capacitance interpoly structure. The nitride layer is thinner than the conventional method and has fewer defects.
Video: How are Microchips Made
