I want to request a quote for the product: ID 444. Type P, 50.8mm silicon wafer. And I want 10 wafers.
This product is supposed to get better Raman Spectroscope background result. If you have any suggestion of selection, please tell me. Thank you.
Silicon Wafers in Research and Production
- Home
- About Us
- Substrates
- Silicon Wafer
- Silicon Wafer Diameters
- 25.4mm Silicon Wafer
- 50.8mm Silicon Wafer
- 76.2mm Silicon Wafer
- 100mm Silicon Wafer
- 125mm Silicon Wafer
- 150mm Silicon Wafer
- 200mm Silicon Wafer
- 300mm Silicon Wafer
- 450mm Silicon Wafers
- 1 Inch Silicon Wafer
- 2 Inch Silicon Wafer
- 3 Inch Silicon Wafer
- 4 Inch Silicon Wafer
- 5 Inch Silicon Wafer
- 6 Inch Silicon Wafers
- 12 Inch Silicon Wafers
- Silicon Wafer Types
- P-Type Silicon
- N-Type Silicon
- Undoped Silicon
- Float Zone
- Silicon Wafer Flats
- Single Side Polished Silicon Wafers
- Double Side Polished Silicon Wafers
- As-Cut Silicon Wafers
- Lapped Silicon Wafers
- Etched Silicon Wafers
- Low Total Thickness Variation Silicon Wafers
- Porous Silicon
- Thin Film
- Ultra-Flat Silicon Wafers
- Silicon Wafer Grades
- Silicon Wafer Mobility Calculator
- Soft Lithography
- PDMS Micro-fluidic Chip Platforms
- Platinized Silicon Wafer
- Epitaxial Silicon Wafers
- Silicon Wafer Surface Roughness
- Silicon Wafer Uses
- Semiconductor and Related Device Manufacturing
- Gold Coated Silicon Wafers
- X-ray diffraction @ zero background specimen holder
- Diced Silicon Wafers
- Wafer Bonding
- Wafer Preperation
- Wafer Processing
- Polyelectrolyte Multilayer Modified Silicon
- Chemical Mechanical Polishing (CMP)
- Silicon Wafer Microfluidics
- Thinnest Silicon Wafers
- Annual Volume of Silicon Wafer Production
- Plasma Etching Silicon Wafers
- Silicon Wafer Annealing
- Direct Radioactive Nuclide Electricity
- Polycrystalline Silicon
- Substrates 2d Materials
- Zinc Oxide on Silicon
- FTIR Undoped Silicon
- High-Pressure Synthesis Experiments
- Silicon Pillars
- PDMS Microstructures
- Silicon Mirros
- Ar Ion Evaporator Deposited Metal Contacts
- Silicon Ingots
- PTFE
- Silicon Wafer Sorting
- Black Silicon Wafers
- Ultra-Flat Silicon Wafers
- Diced Silicon Wafers
- Silicon Wafer Bonding
- Silicon Wafer Fabrication
- Cleaving Silicon Wafers
- Silicon Wafer Orientation
- Ultra-Thin Silicon Wafers
- Custom Silicon Wafers
- Silicon Wafer Suppliers
- Partical Count
- Silicon Wafer Diameters
- Aluminum
- Glass Wafers
- Fused Silica Wafers
- Gallium Nitride Wafers (GaN)
- Germanium Wafers
- Graphene
- Silicon Carbide (SiC)
- Sapphire (Al2O3) Wafers
- Quartz Single Crystal
- Solar Wafers
- TEOS Oxide
- Silicon on Insulator Wafers
- Thermal Oxide
- Silicon Wafer
- Blog
- Wafer Store
- Contact Us
How we Help Researchers
From students and professors in acedemia to scientists in industry, UniversityWafer, Inc. can help. Below is a request for assitance from a PhD candidate.
Reference #176501 for specs and pricing.
Does Silicon Epi Layer Affect Resistivity?
A graduate student need help with the following question
Question:
I'd like to purchase silicon wafers and measure resistivity, especially for the range of 0.01-0.02 ohm-cm using 4 point probe method. Is this resistivity value online measured from the polished surface? and for some wafers(eg. ID 2312), it has specific description (such as "With EPI layer, Hard wetblast/LTO L.M."), would this EPI layer affect its resistivity?
Answer:
A silicon wafer ideally has the same resistivity throught its body. It certainly has the same resistivity on both sides, at the surfaces and within it. Radially there is some variation but for (100) orientation and (0.01-0.02) resistivity range, Radial Resistivity Variation is typically <5%.
YES, you will be able to measure resistivity in the (0.01-0.02)Ohmcm range, both n-type and p-type, with a 4 point probe. It is easiest to measure on wafers with "as-cut" or lapped (but not etched) surfaces. However, if you make good contact, you can measure both on polished and on alkaline-etched surfaces. In the (0.01-0.02)Ohmcm range the measurement is easier than at higher resistivities.
Reference # 201601 for specs and pricing.
What Silicon Wafer Spec is Used as Semiconductor in Pump Probe IR Set-Up?
A Biophysics requested a quote for the following:
I am currently on the lookout for a silicon wafer for use as a semiconductor in a pump probe IR set up, and I see that your silicon product (ID 2441) looks ideal as we were looking for a wafer with a ~200 um thickness. However the diameter is not ideal and I wondered if you could help us with this.
If it is possible we would like this to be cut (diced) into a 25 mm piece. Is this a possibility? If so how much would you charge? This would be ideal as it could then be placed between in our cell whose windows have the dimension of 25.4 mm. This wafer would need to be DSP and I think Prime grade (we would use it for finding time zero).
Reference #206458 for specs and pricing.
How fast Can Silicon Wafers be Delivered?
UniversityWafer, Inc is the leading silicon wafer distributor to universities and research centers internationally. We can delivery next day and if in Boston, same day. Just let us know how fast you need the wafers!
Researcher Testimonial:
The (silicon) wafers have arrived today, and we really pleased with them! Thumbs up to your production crew!
Researcher from University of Exeter
Free Technical Assiatance on All Substrates!
What Silicon-Wafer-Diameters do you Have in Inventory
We have all diameters in inventory. We can also dice any wafer into a dimension or diameter that you need in small and large quantities. Belwo are just some examples of what we carry.
Ultra-thin Silicon 100mm P/B (100) 1-10 ohm-cm 25um 2um thin Silicon also available!
1" Undoped Silicon Wafers (100) >1,000 ohm-cm 250um DSP
2" P-type Boron (100) 1-10 ohm-cm 280um SSP
3" N-type Phosphorus (100) 0.01-0.02 ohm-cm 380um DSP
4" Silicon Wafer Undoped/Intrinsic (100) >20,000 ohm-cm 500um DSP
6" P/B (111) <1 ohm-cm 300um SSP
8" undoped (100) >5,000 ohm-cm 750um SSP
12" P/B (100) 10-20 ohm-cm DSP 850um
We have plenty of silicon wafers at a low price and small quantities of partial cassettes so you can buy less than 25 wafers and as few as one Si wafer.
Silicon Wafer Sale!
We carry a large selection of Silicon Wafers with the following specifications:
Silicon nitride LPCVD and PECVD
Sputtered and Evaporated metals
We can custom make wafers in small quantities. We can dice them, thin them to 2um. We have undoped, low doped and highly doped Silicon substrates that are always in stock.
Typical Client Question regarding silicon wafers:
After looking at your online store, I think we might go with your cheapest silicon wafers, product ID 444. I am in a group that is working on a Senior Design Project to create a biobattery. We need a substrate to pattern with photo-lithography and subsequently deposit various precious metals on that will catalyze certain reactions and conduct electricity. If you have any advice on specific types of wafers we will need for such nano electronic devices I would be happy to know. Thanks.
We make nanomaterials in our lab and one approach is using electrical explosion of wires (EEW). We used one of Scott's old Si wafers (doped with B) and broke off a strip of Si that we attached to electrodes in our EEW apparatus. It worked nicely and we are looking to do the same thing with Ge (Germanium Wafer). We need a wafer that is less than 500 microns thick.
Fill out the form and receive an immediate quote. See bottom of page for recent Silicon Wafers specials.
How do Silicon Wafers make Computer Chips
What do Researchers Use our Silicon Wafers for?
Some clients use the following Si item #447 76.2mm and Si item #1196 100mm silicon wafers for the fabrication of microfluidic devices.
What 100mmm and 200mm silicon wafers are used by researchers
"...to do ini al tests for deep anisotropic etching of diffrac on gra ngs. We have to test different masking material and etch solu ons with these (silicon) wafers. Expected result will be part of a later PhD thesis. After the planned etching the wafers will be not further used and will be disposed.
Silicon Wafer Items Used
- Si Item 2358 - 100mm P-type Boron doped <110> orientation 1-10 ohm-cm resistiivty 500 micron thick Double Side Polished (DSP) Prime Grade
- Si Item #3468 - 200mm Any Type/Dopant <110> Any Res 1000um DSP Mech Grade
What Silicon Wafers are Used for Nanoparticle Formation
"As a (silicon) substrate for nanoparticle formation in ionic liquids. The nanoparticles are for fuel cell investigations."
- Item# 2218 - Silicon 25.4mm P /B <100> ANY 400um SSP
What Silicon Wafer Spec is used for Thin Film Deposition?
- Si Item#978 - 76.2mm P B <100> 1-10 380um SSP Prime Thin Film Deposition
What Silicon Wafers use in the day-to-day Scientific Research?
- Si Item #452 - 100mm P/B <100> 0-100 ohm-cm 500um SSP Virgin Test Grade
- Si Item #453 - 100mm P/B <100> 0-100 ohm-cm 500um DSP Test Grade
- Si Item #589 - 100mm N-type Phosphorous Doped <100> orientation 0-100 ohm-cm resistivity 500 micron thick Double Side Polished
What Silicon Wafers are used for Nanoimprint Processes?
- Si Item #783 - 100mm P/B(100) 1-10 ohm-cm 500um SSP Prime Grade
- 150mm P<100> Any res 650+/-25 um SSP wafers with V notch Prime Grade
- 200mm P <100> Any res 700-750 um SSP wafers with V notch Prime Grade
What Silicon Wafer Coatings are Available?
Thermal Oxide
- Wet Oxide
- Dry Oxide
Silicon (SIN) Nitride
What is Thin Film Deposition on Silicon Wafers
- Sputtering
- E-Beam Evaporation
What are Other Silicon-Wafer Services
- Wafer dicing
- Silicon wafer lapping
- Silicon wafer polishing
- Wafer bonding
What is a Silicon Material Safety Data Sheet (MSDS)
MSDS is just a standard confirmation sheet that show the user the materials properties, how the material should be handled and if it's dangerous.
![]()
What Silicon Wafer Diameters are in Stock?
What Silicon Wafer Dopings are Available?
- Boron doped
- Gallium Doped
- Antimony
- Arsenic
- Undoped also called intrinsic
What is the Thicknesses of Ultra-Thinned Silicon Wafers?
- 2 micron
- 5 micron
- 10 micron
- 25 micron
- 50 micron
- 75 micron
- 100 micron
- 150 micron
- 200 micron
What SOITEC SOI Wafers?
Why pay more for SOI wafers when you don't have to?
What is Silicon's Refractive Index?
Every material that allows electromagnetic waves (light) to pass through it has a refractive index. The index of refraction depends on both the wavelength and the frequency of the light and is given by the equation:
As the wavelength decreases the index of refraction increases. This is because light is absorbed less at longer wavelengths, and more at shorter ones. The refractive index of a material is a complex number and has both real and imaginary components. The real part represents the optical path length change and the imaginary part represents the dispersion loss.
The nonlinear index of Si is very low compared to other semiconductor materials and glass. In contrast, some chalcogenide glasses exhibit several hundred times higher nonlinear index values.
To compensate for this, the spectral response of silicon solar cells is adjusted through temperature compensation. This is achieved by sweeping the sample’s optical path through an array of perforated holes in order to achieve a desired sensitivity.
In this paper we present the measurement and analysis of the optical response of a 6 mm long Fabry-Perot (FPC) silicon etalon to temperature. Using an iterative evaluation we extract the low temperature thermo-optic coefficient (TOP) of silicon from the measured reflected intensity IRIR.
The sensitivity of the sensor to both holes and surrounding material is assessed by analyzing the normalized transmission peak as a function of 1 Refractive Index Unit (RIU) increase for a variety of different hole filling and background materials. Also, the complex refractive indices of Methane, Ethane, and Ethylene generated from their NIST transmission data with a path length of 5 cm are used for comparison purposes.
What are Wafers and How Much Does a Silicon Wafer Cost?
A graduate student requested help with the following quote:
I am a student and I am looking for your academic prices on 3-inch diameter Silicon 100 or 111 wafers (doping level is not important) with a 200-300nm grown or deposited silicon oxide layer. I am looking for 20 such wafers at the lowest price available.
If you are looking for silicon wafers, then you've arrived at the right supplier! We have an large volume of substrates in stock. We don't suffer from the same silicon wafer shortages that have plagued the industry.
The price depends on many factors. Send us your specs and quantity for an immediate price quote!
Scroll right and left for our Customer FAQs Regarding Silicon Wafers!
Fill out the form below for an immediate quote!
We have a large selection of Prime, Test and Mechanical Grade Silicon wafers 1" - 12" Silicon Wafers low doped and highly doped in stock and ready to ship. Examples full and partial silicon wafer cassettes include:
What Is A Silicon Wafer?
Silicon, which is mined from beach sand in only a few places on earth is a natural semiconductor and the most abundant element on Earth except for carbon.
It may not be intentional, but it is possible that most people encounter silicon wafers in their daily lives, or even use them. Most people who live on their devices such as computers and smartphones don't realize that it's silicon that makes all cool tech possible.
![]()
Silicon is the most used element in the electronic device universe. Silicon wafers are much less expensive than other semiconducting materials. Germanium wafers were first used to make semiconductor devices. But a Ge wafers costs about ten times more than comparable silicon specifications. Thus, the humble silicon wafer is the unheralded material changing people's lives.M
During the entire growth process, doping agents can be used to alter the purity of the silicon wafer depending on its manufacturing purpose. These impurities can alter the electronic properties of silicon, which are essential for a wide range of applications depending on the production purpose. In silicon manufacturing, various methods are used to count the number of different types of impurities such as silicon oxide, silicon nitride and thermal oxide.
These degenerate semiconductors can be used as conductors, as they are located in the extrinsic range, which is light and high - doping. They are considered degenerated or extrinsic, depending on whether or not the silicon wafer is added during doping. Silicon doping, which can be added during the growth process, includes aluminum, boron, nitrogen, indium and gallium.
What are Silicon Wafer Applications?
So you have questions about what silicon wafers are used for? And why are silicon wafer used at all? Silicon is the best and most widely used semiconductor, although other conductors are used for more specific applications. Silicon is an excellent option, because its electric current flows through silicon much faster than through any other material, such as copper. So what is a wafer? It's simply a slice of silicon that can be thinner than a piece of paper or as thick or thicker than a hockey puck!
![]() Semiconductors such as silicon wafers can be used to make chips, microchips and electronic devices.
Semiconductors such as silicon wafers can be used to make chips, microchips and electronic devices.
Due to the uniqueness of the electric current in silicon wafers, semiconductors are used to produce ICs (integrated circuits). Ics are the basis for a wide range of electronic devices such as chips, microchips and microprocessors.
Simply put, an integrated circuit is a network of a variety of electronic elements that are brought together to perform a specific function. The silicon semiconductor wafer is the main element in integrated circuits and is surrounded by a layer of semiconductors such as copper, nickel, copper oxide, silicon and other semiconductor materials.
A silicon wafer is a thin disk of semiconductor material that serves as a substrate for a microelectronic device mounted on it. Silicon is the key platform for semiconductors and devices and is used in a wide range of applications that people can only dream of. Although it can be easy to relate silicon wafers to other types of electronic devices such as computers, televisions, mobile phones, and other electronic devices, they are much closer than you might think.
The production of silicon wafers depends on a number of factors, such as the quality of the material, the size (diameter) and the computing power available to the manufacturer.
What is Silicon Wafer Surface Flatness?
What is Silicon Wafer surface flatness? - The thickness variation across a particular area of a polished silicon wafer. For the most part, the flatter the silicon, the better for Photolithography, the process used to make microchips. Generally, a silicon wafer should have less than 1 micron of Total Thickness Variation. However, some are more or less flat than others.
We have Ultra-Flat Silicon with the following spec
Prime Silicon Wafers 100mm P-type /Boron doped <1-0-0> 490-510 micron 0.005-.020 ohm-cm Semi Std Double Side Polished
Total Thickness Variation (TTV) <1 um. These are great for making SOI or MEMS!
The flatness of a silicon wafer is an important attribute of 'round discs'. In the silicon industry, the ![]() more flat the surface, the better it is for forming chips and electronics. Differences in height between wafers are often a cause of contacting problems during subsequent stacking. As such, it is important to check the flatness of a silicon 'round disc' to ensure it is as flat as possible.
more flat the surface, the better it is for forming chips and electronics. Differences in height between wafers are often a cause of contacting problems during subsequent stacking. As such, it is important to check the flatness of a silicon 'round disc' to ensure it is as flat as possible.
The degree of flatness is measured in percentages, and is a fundamental measure of 'round disc' quality. The flatter the surface, the more perfect the 'round disc' is. During the exposure, differences in height may cause defocusing of the backside of the wafer. The flatness of the silicon wafer will influence the depth of focus. A good wafer's surface must be flat from top to bottom.
Flatness is an essential metric for the quality and reliability of a silicon wafer. The surface of a silicon wafer is crucial for the efficiency of the device production process. In the semiconductor industry, the quality of a silicon wafer will affect the performance of a semiconductor. In order to ensure that a silicon wafer is reliable, it must be flat. And the flatness of a silicon wax is a good indicator of the quality of a product.
The flatness of silicon wafers is a crucial quality parameter. High-quality silicon wafers are highly resistant to corrosion. As long as they have a high level of flatness, they can withstand many processes. For example, a single layer of metal may be sensitive to heat. The thickness of an individual wafer can vary by more than a hundred times, which can cause short-circuits and errors.
In addition to flatness, the thickness of a silicon wafer's surface can also be affected by its bow. The bow is a measurement of the distance between the front and back surfaces. A shallow bow means that the edges of a silicon wafer are curved. The thickness of a silicon wafer can influence the performance of the device. The more rounded it is, the higher its flatness.
The surface flatness of a 'round disc' is defined as the degree of flatness. The flatter it is, the better. The higher the surface is, the more perfect the wafer. If the surface is too rough, it can cause problems with subsequent stacking. The difference in height is a characteristic of a 'round disc'. If the surface is uneven, it may cause a 'bumpy'.
The surface flatness of a 'round disc' is measured in mm. The flatter the surface, the better. A smoother surface is ideal for microchip production. A rougher surface will cause contacting problems. Consequently, the more even the surface, the better. Moreover, a higher flatness will help you make the smallest silicon wafer possible. You'll be amazed at how many possibilities you have when you have a clean, sanded, and polished silicon wafer.
Silicon wafers must be flat to meet strict requirements. The surface flatness of a silicon wafer determines its quality and reliability. Similarly, a defective silicon can compromise high-tech systems. This is why the best quality silicon wafers will have maximum flatness, or fatness, of about one to three microns. This is a very low amount of inconsistency.
The surface flatness of a silicon wafer is a critical factor in semiconductor manufacturing. The flatness of a silicon wafer must be smaller than the depth of focus of optical lithography exposure tools. The standard thickness of a silicon wafer is a requirement for the most efficient production of semiconductors. The thickness of the wafer is measured in microns. If the thickness is too thin, the wafer is considered thick.
Silicon Wafer Flat Surface Documentary
https://www.youtube.com/embed/2cZ0XJWxyH0
How to Measure a Silicon Wafer Surface Roughness
The first step in measuring a silicon wafer's surface roughness is to understand the process involved in its polishing and cleaning. Aiming for a low level of roughness is important to ensure that the device's circuits will operate properly. However, excessive roughness will damage or destroy thin layers such as the gate oxide and tunnel oxide. This process is also important in other electronic materials such as SiC, GaAs, and GaN.
There are several methods for evaluating a silicon wafer's surface roughness. AFM is one of the ![]() fastest and most accurate ways to measure surface roughness. AFM uses a high-resolution optical-power meter to evaluate the intensity of a laser beam. This technique uses an acoustic wave to create a high-resolution image of a sample surface.
fastest and most accurate ways to measure surface roughness. AFM uses a high-resolution optical-power meter to evaluate the intensity of a laser beam. This technique uses an acoustic wave to create a high-resolution image of a sample surface.
Another method is to use a confocal microscope to examine the surface roughness. This method has a wide spectral range, which makes it ideal for assessing the surface of low-K materials. Infrared laser measurements can reveal the roughness of silicon wafers. Sharper AFM tips reveal finer features and can lead to a greater effective roughness, which can be used to estimate the surface of a silicon wafer.
Using an acoustic microscope to measure a silicon wafer's surface roughness, the confocal microscope is an excellent tool for studying low-K materials, such as Si wafers. AFM is extremely accurate, with high lateral resolution. The confocal microscopy is an indispensable tool for research and development. It is also quick and easy to use.
One of the most popular methods for measuring a silicon wafer's surface roughness is to use a confocal microscope. The AFM tool is useful in studying the surface roughness of Si and other low-K materials. The higher the sensitivity of the scanner, the greater the effective roughness. This technique is a great way to assess surface roughness and porosity of low-K materials.
The new technology is based on the same principle used to measure other types of materials. AFM uses a laser to determine a surface's roughness. Infrared light is transmitted through a single-crystal silicon, and is therefore reflected as an infrared laser. A chamfer can be measured by measuring the intensity of the light. The higher the infrared intensity, the higher the surface roughness.
AFM is an excellent tool for studying silicon wafer surfaces. It can also be used to determine the porosity of low-K materials. AFM uses an infrared laser to measure a silicon wafer's surface roughness. Unlike conventional methods, this method requires no special equipment. AFM is an excellent tool for studying the surface of low-K materials. It can be used for a variety of applications and can be used in any industry.
The use of an infrared laser allows for measurement of the surface roughness of silicon wafers. This technique is particularly useful for studying low-K materials. It has been shown that the sharper the AFM tip, the larger the surface roughness. The effect is illustrated in the following figure: a. The infrared lasers are used to measure the surface roughness of silicon wafers, but they are not as effective as the optical-powered ones.
Scattering light is a popular way to measure a silicon wafer's surface roughness. The technique uses a confocal microscope to analyze the surface of a silicon chip. AFM can also be used to measure low-K materials. By contrast, the more refined the AFM tip, the more accurate the roughness measurement will be. The infrared laser method has a high level of accuracy and is used for research and industrial applications.
AFM is a technique that allows the measurement of silicon wafers. It uses a special probe that measures the surface of a silicon wafer. These sensors are sensitive enough to detect the tiny changes in the surface of the wafer. When a silicon wafer is prone to cracking, it is best to avoid these types of defects in the first place. AFM can also be used to detect the defects in a semiconductor.
What is the Roughness Value (rms) of your Silicon Wafers?
The majority of our Prime Grade wafers have a roughness value Ra<5Å . We can provide roughness specifcation upon request.
What is the definition of silicon wafers?
A silicon wafer, or substrate, or silicon is grown in a tube from a seed into a long ingot that is then sliced into various thicknesses used in electronics for the fabrication of integrated circuits and in photovoltaics. The wafer serves as the substrate for microelectronic devices built in and over the wafer and undergoes many microfabrication process steps such as doping or ion implantation, etching, deposition of various materials, and photolithographic patterning. Finally, the individual microcircuits are separated (dicing) and packaged.
Can you Deposit Platinum (Pt) on Silicon Wafers?
Yes! We sell Platinised and thin films of almost all the metals! Just let us know the specs and quantity for an immediate quote!
How to Deposit Platinum on Silicon Wafers
The deposition of platinum on silicon wafers is a challenging task. While the element is good at bonding with other elements, its affinity for oxygen and the silicon substrate limit its use in electronics. The following procedures describe how to deposit platinum on silicon wafers. You can learn more about the process in the sections below. Let's have a look at a few of the most common methods. You can find more information about the methods by reading on.
The DC/RF magnetron sputtering technique is a popular way to deposit platinum on silicon wafers. This technique involves depositing a thin film of platinum on an insulating oxide layer. The process then requires an inert or oxidation atmosphere for platinum deposition. The silicon wafer is then annealed at a certain temperature range to remove any impurities in the layer.
A number of methods have been developed to deposit platinum on silicon wafers. The two most common processes are sputtering and electron beaming. The first two methods use a hydrogen-based plasma to deposit the metal. These techniques use an oxidation-free plasma and oxygen-free inert atmosphere. After the platinum is deposited, the silicon substrate is annealed to a temperature in the range of 550°C.
The other method is to deposit platinum on silicon wafers by adding oxygen to them. A conventional platinum-oxide process uses a hydrogen-free gas to deposit the metal on a silicon wafer. The oxygen-containing oxygen reacts with the platinum-oxide layer to form Pt+Si. The reaction takes around 20 seconds at 450 degrees Celsius. It is then deposited on the silicon wafer in an inert atmosphere.
The platinum on silicon is deposited on silicon wafers by using a plasma-based method. This process is known as direct plating. After the process, the platinum will deposit on the silicon wafers. Once the platinum is deposited, the entire process is then controlled to yield the desired PtSi. A high-quality PtSi film is achieved after all of the heat treatments. The present invention is a method of forming a Pt thin film on silicon.
The process of forming the Pt+Si on silicon wafers is a process known as vapor phase deposition. In this method, platinum is deposited on the silicon wafers after a chemical treatment at 450 degrees C. This process produces a thin layer of Pt on the silicon, and the Pt on the silicon is called "deposited" in the present invention. This step is the only method that has demonstrated the ability to deposit a Pt metal on silicon in such a manner.
Several steps are necessary to deposit platinum on silicon wafers. A few experiments show that, at 200 degrees C, Pt can form a Pt-Si layer. However, this process is not complete at that temperature. The resulting film of Pt on silicon is too thin to undergo full transformation. In order to remove oxygen, the Platinum on the silicon substrate is deposited in an inert atmosphere.
The first step of this process is to apply a negative bias on the silicon wafers. The resulting metal is then deposited on the silicon. The process results in a thin layer of Pt on the silicon. The next step is to introduce oxygen in the metal to the platinum layer. The process is repeated until the desired thickness is reached. If the thickness is below the desired level, the platinum is deposited by thermally treating the silicon wafers at 450 degrees C.
In order to deposit Pt on silicon, a negative bias is used to deposit Pt on silicon wafers. A low-bias silicon wafer will not allow oxygen to penetrate the wafers. Instead, it will form a platinum thin film on the silicon substrate. When this occurs, the Pt is bound to the silicon, which will prevent the metal from oxidizing. A positive bias will prevent this.
There are several ways to deposit platinum on silicon wafers. In one method, it is done under an oxidation atmosphere. The second method is done in an inert atmosphere. In this method, the platinum is deposited onto the silicon. The oxygen in the platinum layer is sputtering on the silicon wafers. When the electrode is prepared, it will be deposited on the silicon wafer.
How Do You Clean Silicon Wafers?
The first step in cleaning Silicon wafers is solvent cleaning. Solvents can be very effective in eliminating organic impurities, but they often leave a residue on the surface of the silicon. A typical solvent used for this purpose is acetone, which is used to restore the substrate material. Using a solution of one part acid to four parts water, silicon wafers are immersed in the solution for two minutes. After the bath, the wafer is rinsed with deionized water. Once the silicon has been thoroughly cleaned, a wettability test is conducted to confirm that no contamination is left on the surface.
Another popular method for cleaning silicon wafers is Piranha etching. This process involves applying ![]() large amounts of sulfuric acid to the wafer substrate. This solution is highly effective in removing photoresist, nitrates, and other organic materials. The standard mix for a Piranha etch is three parts of sulfuric acid to one part thirty percent hydrogen peroxide, but there are other protocols that use higher concentrations. After the process is completed, the wafers are typically water-compatible.
large amounts of sulfuric acid to the wafer substrate. This solution is highly effective in removing photoresist, nitrates, and other organic materials. The standard mix for a Piranha etch is three parts of sulfuric acid to one part thirty percent hydrogen peroxide, but there are other protocols that use higher concentrations. After the process is completed, the wafers are typically water-compatible.
During a silicon wafer cleaning process, the surface is cleaned using a solution containing ammonium hydroxide and hydrogen peroxide. Then, the silicon is soaked for 15 minutes in the RCA bath solution. Afterwards, the wafer is rinsed with DI water and placed under a stream of water. After the rinsing, the silicon is rinsed in DI water.
Cleaning silicon wafers involves a combination of wet and dry methods. The most common method is the wet cleaning method, but there are some advantages to dry cleaning strategies, too. The wet cleaning method works best for glass-free surfaces, while the dry method is best for metal-free silicon wafers. If the silicon is made of silica and has a smooth surface, then wet cleaning is an effective solution.
There are several different ways to clean silicon wafers. However, the most popular method is the RCA clean. This method removes organic debris from the silicon by adding 5 parts water to 30% hydrogen peroxide and 27% ammonium hydroxide. The RCA cleaning method leaves a thin layer of oxidized silicon on the surface. This is a more effective way to clean silicon than the previous methods.
The RCA bath is another common method of cleaning. In this method, an acetone bath is mixed with water and ammonium hydroxide. The silicon wafer is soaked in this solution for about 15 minutes. After the RCA bath, the wafer is removed and rinsed in DI water. Then, the bath is replaced with DI water. The RCA solution is used to clean the silicon.
The RCA process is the most common method of cleaning silicon wafers. It uses a mixture of water and hydrogen peroxide to clean the silicon. This method is the most effective because it leaves a thin layer of oxidized silicon on the surface. It is also more expensive than RCA, but it is worth it if you're considering using it. It's a good way to avoid the contamination of the silicon wafers.
RCA baths contain a mixture of hydrogen peroxide and ammonium hydroxide. The silicon wafer is soaked into the solution for about fifteen minutes, and then rinsed in DI water. Then, the wafer is removed from the RCA bath under flowing water. This method is also known as the RCA bath. This process has many advantages. Moreover, it is a proven method.
Cleaning silicon wafers is not an easy task. They are highly sensitive and can become contaminated easily. Unlike other materials, these materials are not affected by oxygen. The acetone baths will keep the silicon clean. During the process, the acetone bath will be heated to 55 degrees Celsius. This will help in cleaning the silicon wafers. There are many types of a silicon bath.
RCA clean is a common process for cleaning silicon wafers. It is a standard step that is required before high-temperature processing. It involves the use of a solvent at a temperature of about 80 degrees. The solution will remove organic residues and particles while changing the zeta potentials of the particles. The RCA clean process will leave the silicon with a thin layer of silicon dioxide and some metallic contamination.
How do you clean Si 100 wafer before silicon dioxide is formed for bump production?
The RCA clean is a standard set of wafer cleaning steps which need to be performed before high-temperature processing steps (oxidation, diffusion, CVD) of silicon wafers in semiconductor manufacturing.
Werner Kern developed the basic procedure in 1965 while working for RCA, the Radio Corporation of America.[1][2][3] It involves the following chemical processes performed in sequence:
Removal of the organic contaminants (organic clean + particle clean)
Removal of thin oxide layer (oxide strip, optional)
Removal of ionic contamination (ionic clean)
Can you resize Silicon Wafer diameters from 200mm to 100mm?
Yes! We can laser down the wafer so you could get two 100mm from one 200mm wafers including flats!
![]()
Below is a wafer lasered. Send us your diagram and specs for an immediate quote.
What is Silicon-Wafer Reclaim?
Semiconductor companies reclaim silicon wafers to save money. Below is a reclaim quote request from our client.
"I am looking to buy 3in Si wafers for optical monitoring (mainly vis-NIR ellipsometry) purposes. What we currently have are single side polished P(100) wafers. Resistivity is not important for us. We are also looking for 1in Si wafers for the same purpose and same specifications. Finally, we also want 3in cover wafers. I was wondering if you sell ‘reclaimed’ or re-used Si wafers, which I think could be good for this purpose. I wanted to check the price and potential lead time directly with you. We would need 25 of each of the 3in wafers mentioned above and maybe 100+ (renewable) of 1in Si wafers, if you have in your inventory."
UniversityWafer, Inc. Quoted:
- 3in Si wafers for optical monitoring (mainly vis-NIR ellipsometry). single side polished P(100) wafers.
- 3in cover wafers. ‘reclaimed’ or re-used Si wafers delivery
- 1in Si wafers for optical monitoring (mainly vis-NIR ellipsometry). single side polished P(100) wafers.
Wafers with thin films or oxide etc on their surface are stripped and cleaned so the wafers can be reused. Often companies that want to save money or protect their intellectual property will reclaim their wafers.
The process of Silicon-Wafer Reclaim involves processing silicon wafers after the ![]() manufacturing process is completed. The reclaim process can range from a "strip and clean" process to a "full repolish." It is crucial to understand which process is right for your product. Listed below are some of the most common types of reclaim: lithography, polishing, and annealing.
manufacturing process is completed. The reclaim process can range from a "strip and clean" process to a "full repolish." It is crucial to understand which process is right for your product. Listed below are some of the most common types of reclaim: lithography, polishing, and annealing.
Problems Reclaiming Silicon Wafers
Reclaiming silicon wafers may be difficult and the yield may be low as coatings and scratches may make the wafers un-recyclable. The quality of the raw materials and systems used in the reclaimed silicon wafers is important. A company should have "customer equivalent or better" technologies to satisfy semiconductor device manufacturers' requirements. ASP standards are very strict, and the process must be carried out with utmost precision so that the reclaimed silicon wafers must be as close to the original as possible.
What are the Silicon Reclaiming Steps?
Reclaimed silicon wafers are reprocessed through multiple stages. The first step in the process is examining the wafers. Once screened and inspected, the wafers go through lapping and etching. The second phase of the process is repolishing. This depends on the diameter of the silicon-wafer and the specifications. The final step is cleaning and inspection of the reclaimed wafers.
- Cleaning
- Lapping
- Grinding
- Polishing.
Reclaimed silicon wafers will be thinner after reclaiming. The amount of silicon removed depends on many factors.
How Big is The Silicon Wafer Reclaim Market?
The silicon wafer reclaim market is highly fragmented based on its application. The market is segmented by type, such as solar cells, and then by geography. The solar cells segment is expected to dominate the market during the forecast period. The reclaim technology also reduces costs of manufacturing a variety of components, including those in integrated circuits.
The market for Silicon-Wafer Reclaim is highly competitive. To be profitable, reclaim wafers must have the highest yields. In order to remain competitive, manufacturers must be able to make good use of reclaim wafers. The reclaim process involves reprocessing and refining a wafer. For example, reclaimed silicon wafers must be fabricated in factories with a high degree of precision.
Where to buy Silicon Wafers?
Which keyword did you use to find us? Tell us and you'll receive a discount on your order! For more easy to access information visit our partner.
- silicon price
- si wafer vendor
- silicon producer
- wafer production
- silicon producers
- mono silicon
- wafer bonding
- silicon wafers definition
- silicon wafer manufacturers
- silicon wafer processing
- si wafer orientation
- si wafer flat
- si wafer resistivity
- silicon wafers for solar cells
- si wafer cleaning
- si wafer thinning
- silicon wafer orientation
- 4 inch silicon wafer dimensions
- scrap silicon
- 4 inch silicon wafer
- Boron Doped Silicon
- Czochralski Process
- Semiconductor Wafers
- silicon wafer size
- wafer
- wafers
- silicon wafer
- semiconductor wafer
- what is wafers
- what is wafers
- silicon wafers
- wafer definition
- what is a wafer
- wafer semiconductor
- semiconductors wafer
- cpu wafer
- wafer meaning
- silicon boule
- what are wafers
- chip wafer
- chip wafer
- wafer chips
- wafer chips
- wafers computer
- wafer electronics
- define wafer
- wafer manufacturing
- si wafer
- 300mm wafer
- silicon substrate
- wafer silicon
- wafer silicon
- what is wafer
- silicone wafer
- silicone wafers
- how are silicon wafers made
- silicon wafer thickness
- silicon wafer manufacturing
- computer wafers
- microchip wafer
- semiconductor wafers
- wafwr
- wafwr
- wafer chip
- wafer chip
What Substrate Material Should I use for Coating Research?
UniversityWafer, Inc. can help you find the right wafer for all your research.
Clients have used the following Si Wafer for the coating research:
Si Item #1196 - 100mm ANY TYPE, DOPANT, ORIENTATION 500um SSP MECHANICAL GRADE
UniversityWafer, Inc. and our partners manufacture pure silicon with electronic properties that ranges the full spectrum of specs and diameters from 25.4mm to 300mm. Diced wafers are also available upon request.
What is Float Zone (FZ) Silicon?
We have a large selection of float zone silicon (also called undoped silicon wafers or intrinsic) in stock and you can buy as few as one wafer online!
A University Researcher requested the following quote:
I am interested in the following float zone, not Czochralski silicon wafers with the following specs:
- double-side-polished
- p-type-doped to resistivity of 3-6 ohm-cm
- 300 nm oxide
we typically work with 4” wafers but diameter is not important. Thickness, again typically we use 500 microns but it is not too important; anywhere from 300-600 microns would be fine. Orientation is also not important, I think we typically use 100 just since that’s what’s available. Quantity: between 10-20, depends on price. Can you grow oxide on any of the Si wafers listed on your webstore? So I don’t have to choose from only the “oxide” wafers?
Some of the Si wafers are labeled float zone. I’m looking for wafers of that intrinsic quality, but which have then been doped to the few ohm-cm level. Is that done? Or are doped wafers only CZ?
Since we’ll use this material for experiments on infrared transmission, the resistivity is important: there’s a narrow range where the wafers are low-doped enough to be transparent to IR, but highly doped enough to remain conductive at low temperatures.
any chance you have something like this?
Please reference #224535 for specs and pricing.
Float zone silicon is a high-purity form of silicon obtained by a vertical zone melting process. It is a  superior alternative to the Czochralski process because the molten silicon contains sufficient surface tension to prevent charge separation. The float-zone process produces very low levels of light impurities and nitrogen helps control micro-defects and improves the mechanical strength of the wafers.
superior alternative to the Czochralski process because the molten silicon contains sufficient surface tension to prevent charge separation. The float-zone process produces very low levels of light impurities and nitrogen helps control micro-defects and improves the mechanical strength of the wafers.
Float zone silicon is an extremely pure form of silicon that is produced through a process called vertical zone melting. Its advantages over Czochralski silicon include low oxygen and carbon impurities. Its characteristics make it ideal for power devices, solar chips, and RF circuits. These properties make float zone silicon an ideal material for many types of devices. What is a Float Zone Silicon Wafer?
Float zone silicon is a high purity silicon fabricated using vertical zone melting. Its advantages over Czochralski silicon include low oxygen and carbon impurities. Float zone high-purity make it an ideal substrate for power devices, solar chips, and RF circuits.
Ask Us About Boron Phosphide Semiconductors
We can customize boron phosphide for your research of high-power, high-frequency devices as well as laser diodes.
Boron Phosphide Semiconductors
Single crystal growth of boron phosphide (BP) semiconductor layers has been reported. The single crystals are grown using a high pressure flux method or a chemical vapor deposition process. The electrochemical properties of BP single crystals are promising photocathodes. In addition, BP contains autodoped silicon in a concentration of 1018 to 1020 atoms/cm3 where Si atoms are acceptors and incorporated into phosphorus sites. The lattice constants of BP are determined by the Bond method.
In epitaxial growth, a thin layer of boron phosphide is formed on a substrate. This substrate is heated to a high temperature and is then in contact with the reaction gas. This process results in a material that has excellent electrical and crystallographic properties. The semiconductor is then fabricated onto a chip. The device can be used directly, or it can be made into an integrated circuit.
The impurity element is a chemical compound of the VIb group. Among the impurities, sulfur, tellurium, and selenium are commonly used. The impurity source is diluted in carrier gas, and then is deposited on the substrate. The impurity element is then charged into the boron phosphide during the vapor phase growing process.
In epitaxial growth of boron phosphide, the organic compound is cooled to a liquid phase, and the amount of the organic compound is controlled by varying the flow rate of carrier gas. The resulting layer has a mobility of 50 cm 2 /V sec. The film thickness is controlled using a diborane-diode process. The diborane can also control the thickness of the boron phosphide film.
The growth of boron phosphide semiconductors is a multistep process that involves two steps. The diborane is used as the carrier gas, which forms a thin film. The film thickness of the boron phosphide semiconductor can be controlled by controlling the amount of impurities. Further, the diborane increases the mobility of the phosphorus.
The boron phosphide semiconductor layer formed by a process involving diborane is a P type film. It has excellent crystalline structure, a high mobility of 50 cm/V/sec (1m thick), and a low thermal conductivity. This compound is a good choice for the fabrication of transistors and other electronic devices. The thin layer is easily formed by the process, which uses hydrogen as a carrier gas.
A layer of boron phosphide semiconductor is composed of a single crystal. In a typical monocrystalline boron phosphide, the silicon crystal is a P type semiconductor. In a P type semiconductor, the phosphorus phosphide film is characterized by an excellent crystalline structure. The mobility of a polycrystalline thin film of boron phosphate is 50 cm/Vsec, which is very high for a one-micrometer thick layer.
The semiconductor layers of boron phosphide are characterized by crystalline structures. A P type boron phosphide layer has a high mobility. Reflecting electron diffraction reveals the presence of Kikuchi bands. The mobility of a phosphorus semiconductor layer is similar to the mobility of a silicon. The crystalline structure of a boron phosphide film is 100 nm thick, which is suitable for a single-layer transistor.
The boron phosphide semiconductor layer is a single crystal that is composed of phosphine (PH 3), diborane (B 2 H 6), and phosphorus. The BP/Si composite substrates have a high surface area and are good for semiconductor devices. Its thickness ranges from 500 to ten thousand atoms. Several methods have been developed for the fabrication of boron phosphide.
The present invention provides a novel method for epitaxial growth of boron phosphide semiconductor layers on single crystal substrates. It is based on a technique that uses hydrogen, phosphorous, and boron as reducing agents. For example, the boron phosphide layer is prepared by a solvent, which contains bromide and nitrogen. Once this reaction is completed, a thin film of phosphorus is produced.
The BP/Si composite semiconductor substrate consists of a silicon substrate with a boron phosphide deposited on it. The BP/Si technology has the potential to create ultrahigh-speed and low-power electronic devices. This method allows for a wide range of spectral and optical properties. The BP/Si process is a low-cost and environmentally friendly way to make a variety of phosphorus semiconductors.
https://www.youtube.com/embed/FZ43S99R56U
What Silicon Wafers Used in Photolithography
Researchers have used the following substrates with great success!
Si Item #809 - 100mm N/P <100> 1-10 ohm-cm 500um SSP Prime
What Silicon Wafers Are Used in Photolithography? The term "photolithography" is defined as the technique of using light to form patterns on the surface of a wafer. This process is used in the fabrication of integrated circuits by removing protective layers and exposing the areas where chemical reactions can take place. To learn more about photolithography, you can view the Glossary or watch a video about the process.
Initially, photolithography was used to fabricate printed circuit boards. During the early 1950s, the 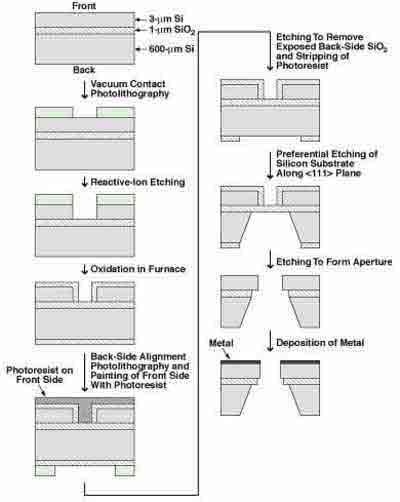 techniques were adapted to produce finer designs on silicon wafers. In particular, Frosch and Derick developed a silicon-dioxide layer, which helped them achieve their goal of creating a hydrophobic surface. These two discoveries proved to be a game-changer, as they were able to produce n-type and p-type semiconductors using only one layer of silicon.
techniques were adapted to produce finer designs on silicon wafers. In particular, Frosch and Derick developed a silicon-dioxide layer, which helped them achieve their goal of creating a hydrophobic surface. These two discoveries proved to be a game-changer, as they were able to produce n-type and p-type semiconductors using only one layer of silicon.
The next step in photolithography is the preparation of the silicon wafer. The process begins with a thin layer of silicon oxide on the surface of the wafer. A layer of silicon-dioxide is then applied to the surface of the silicon wafer, which promotes the adhesion of the photoresist. The silicon-dioxide layer is made from a polar compound, which is applied to the silicon-dioxide layer. During the exposure to light, the layers of silicate crystals develop into a thin film.
Various photoresist compounds are used to create a mask on the surface of the silicon wafer. This allows the developer to control the doping and etching process. There are three major components of photoresist: the liquid photoresist, the xylene solvent, and the HMDS. The latter is applied on the silicon wafer before exposing the silicon. After the exposure, the silicon polymerizes, resulting in a high-quality image.
To prepare the silicon wafers for photolithography, researchers must first prepare the silicon-dioxide layer. HMDS is a hydrophobic layer that helps the photoresist adhere to the silicon surface. The negative photoresist is also called a negative photoresist. Both of these types of photoresists are used in photolithography. However, the positive resist is better than the negative one.
HMDS is applied to silicon wafers to enhance the photoresist's adhesion. HMDS is a polar compound that is applied to the silicon wafer in liquid form. The polar photoresist is applied to the silicon, which will then allow it to be etched. The process also involves the use of a negative photoresist. A negative resist will not stick to the silicon, but the photoresist will not.
The photoresist is a compound that can be applied to a silicon wafer to control etching and doping. The HMDS compound is applied to the silicon wafer in a liquid form. When exposed to light, the chemical reacts with the photoresist to produce a mask. The result is a film that can be etched and has a micro-scale feature.
In the most basic sense, photolithography is the process of applying a photoresist to a silicon substrate. The photoresist is the material that controls the thickness of the photoresist. It restricts the width and depth of the topography on the wafer. The negative photoresist has better adhesion and is therefore the preferred type for small and medium-sized features.
A photoresist is a polymer that is composed of a polymer matrix and a solvent. This photoresist reacts with light and forms a pattern on the silicon wafer. A negative photoresist will be more resistant to the etchant than a positive one. It will also have better adhesion to the silicon wafers and is the preferred method for small features.
To make photolithography work, silicon wafers are used. The silicon wafers are coated with a photoresist. The process requires a thin layer of novolac resin to make the photoresist visible. The thin film will then be exposed to the light. It will not be seen until the silicon is polished and the light-sensitive layers are exposed to the photoresist.
What Silicon Wafers Used to Make Microfluidics Molds
Scientists have used the following for their microfluidics research.
Si Item #783 - 100mm P/B <100> 1-10 ohm-cm 500um SSP Prime Grade
How Silicon Wafers Are Used to Make Microfluidics Molds
Microfluidics is an emerging technology that uses microfluidic devices to create complex systems. This technology can be a game changer for the field of biomedical research. For example, researchers at the Wyss Institute have developed human "Organs-on-Chips," which can be sliced and inserted into devices that can perform genetic testing. The advancement could eliminate the need for animal testing.
In order to make these microfluidic devices, manufacturers have begun by making molds of ![]() transparent silicon wafers. The silicon wafers were mounted on a thin metal frame using dicing tape, which had a sticky backing. The wafer was then diced by a dicing saw to create channels of varying widths. The channel width is set at a pixel-wide 1mm, 500 mm, 300 m, and 200 m.
transparent silicon wafers. The silicon wafers were mounted on a thin metal frame using dicing tape, which had a sticky backing. The wafer was then diced by a dicing saw to create channels of varying widths. The channel width is set at a pixel-wide 1mm, 500 mm, 300 m, and 200 m.
Then, the silicon wafer was mounted onto a piece of dicing tape. The dicing tape was then applied to a thin metal frame to hold the silicon. Then, the wafer was diced with a dicing saw. Then, a channel was cut in the silicon wafer with a 0.5 mm width. After that, a slicing saw sliced the wafer.
Then, the silicon wafer was mounted on dicing tape. This tape has a sticky backing and holds the silicon on a metal frame. The dicing saw then diced the silicon. The channel widths were 1mm, 500 m, 300 m, and 200 m. Each channel is made in a vacuum. The mold was then prepared for the microfluidics fabrication process.
These devices are made with a wide range of materials. Most of them are transparent for optical observation. Fortunately, they are also biocompatible for life science applications. The silicon wafers used to make these devices are etched on the surface of the wafer with a specific pattern. In the process of manufacturing the molds, the silicon wafer was mounted on a dicing tape. Then, the dicing saw diced the silicon substrate. The channel widths were set to one mm, three hundred m, and 200 m.
The process used to create these molds was similar to that used for making microfluidics. The silicon wafer was mounted on a dicing tape. The dicing saw is a sticky tape, so it stuck to the glass or metal frame. Once the mold was ready, it was sliced with a dicing saw. Once the chip was diced, it was then bonded to the mold.
The main differences between silicon and PDMS are the materials used for the molds. Both types of materials have different applications. For instance, a transparent material is preferred for optical observation. A biocompatible material is needed for life science applications. The process used for microfluidics molding involves two processes. First, the silicon wafer is mounted on a dicing tape. The dicing tape sticks the silicon to a thin metal frame. Second, the silicone wafer is then diced with a dicing saw. Third, the dicing tape is removed from the glass.
PDMS molds can be made from a variety of materials. PDMS is a good choice for microfluidics made from polymer. The polymer is porous and is suitable for the production of monolithic separating columns. These are also flexible and can be used in the manufacture of microfluidics. They are also incredibly versatile.
PDMS is a polymer used to make microfluidic devices. The low UV range of PDMS allows for a variety of materials. It is also suitable for building monolithic separating columns. In addition, it is a good material for microfluidics. You can also find PDMS molds on eBay. They are available on the Internet.
The mold masters are made by patterning a photosensitive resin with a photomask. The photomask has the design of the microfluidics mold. The PDMS replica is then cured at 70 degrees Celsius before being peeled from the master mold. Overall, the process can take up to 24 hours. However, there are several advantages to using PDMS.
What Silicon Wafer is Used in Heterojunction Device Research?
Lab researchers have used the following double side polished silicon wafers to fabricate heterojunction devices.
Si Item #775
100mm P/B <100> 1-10 ohm-cm 500um DSP
Heterojunction devices are made from two types of silicon: p-type and n-type. The p-type is a semiconductor with a high-density structure, while the n-type has a low-density structure. The difference between the two types of semiconductors is the level of heterojunction. The p-type is a type of polysilicon, and the n-type is a polysilicon. Its n-type structure means that the n-type transistor has a higher current capacity than the monolithic silicon devices.
This structure can be used in heterojunction devices. A higher bulk resistivity can be useful for solar cell architectures with good surface passivation. A recent study produced silicon heterojunction solar cells made on very high bulk resistivity wafers, with PCEs that were comparable to those of commercial bulk resistivity wafers under different illumination conditions. Moreover, the high resistivity wafers had a higher breakdown voltage, which means they were more reliable than standard silicon wafers.
A higher bulk resistivity is beneficial for silicon heterojunction device research in many applications. The high bulk resistivity wafers used in this research showed high performance under various lighting conditions and at higher temperatures than those of standard bulk resistivity wafers. They also had higher breakdown voltages than the commercial bulk resistivity wafers. This is a big step in the field of solar energy.
Heterojunction devices are made of silicon semiconductors with high bulk resistivity. The high bulk resistivity silicon wafers have been used for solar cell research. In addition to being more reliable than standard bulk resistivity wafers, they also had a higher breakdown voltage than standard bulk resistivity. The high bulk resistivity of these semiconductors made them more attractive for heterojunction devices.
It is also possible to use the silicon heterojunction as a base-emitter junction of a bipolar transistor. The high electron mobility silicon transistors have high forward and reverse gains. The transistors used in these devices have low leakage currents. These devices are also compatible with different illumination conditions. They are both known as "bipolar" heterojunction" and are highly effective in a variety of applications.
The highest bulk resistivity silicon wafers have high open-circuit voltage, which is important for solar cells with low open-circuit voltage. The high open-circuit voltage is an additional benefit for HJT devices, since it mitigates the negative effects of hot temperatures. In contrast, thin c-Si cells are difficult to mass produce, and this makes the heterojunction architecture an attractive choice for solar cells.
A high bulk resistivity silicon wafer is an advantage in solar cell research. Its high bulk resistivity will result in a lower breakdown voltage, compared to the standard c-Si. In addition, it will be more durable than standard bulk resistivity wafers. A hydrogenated amorphous silicon wafer will last much longer than a regular silicon wafer.
The high bulk resistivity silicon wafers are preferred for a number of reasons. The low-bandgap silicon wafers allow for higher-voltage operation, whereas c-Si will only be suitable for single-layer semiconductors. They can be etched more easily and have higher breakdown voltages, but they are expensive. The high resistivity wafers can withstand the high temperature conditions that occur in solar cell research.
In addition to its high-bandgap-bandgap-bandgape silicon wafers are also used in HJT cell research. They are made with the amorphous silicon that is used in the solar cells. This is a thin, flexible silicon wafer that is not only high-bandgap but also has a low bulk-bandgap-bandga-gap-switch.
In addition to its high-band-gap semiconductors, heterojunctions are a good way to improve laser efficiency and control costs. The two types of heterojunction devices are similar in design but differ in the process of manufacturing. However, the technology behind heterojunctions can be improved using nanoscale silicon-based devices. In some cases, they are even a lot cheaper to produce than their conventional counterparts.
What are Silicon Wafers Used For?
Silicon wafers are made from a material called silicon. They are the main ingredient in semiconductors, which are used in all kinds of electronic devices. Because of their high density, silicon is the second most abundant element in the universe, and their versatility and cost-effectiveness make them an invaluable resource in technology. However, what are some of the applications of silicon? Let's take a look at some of the most common uses.
One of the most common uses of silicon is in the production of semiconductors, which are used to create electronic devices. In addition to semiconductors, silicon wafers can be used for many other applications. In the semiconductor industry, these materials are used to make tools for the manufacturing of electronic components. A dielectric etching system uses a plasma to remove conductive components. This process is known as reactive ion etching, and it involves bombarding the surface of a silicon wafer with charged particles.
The most common application of silicon wafers is in the fabrication of semiconductors. Aside from electronics, silicon is the primary ingredient in integrated circuits, which are electronic gadgets. A silicon wafer is a thin slice of this material that acts as a substrate for microelectronic devices. Examples of silicon wafers include smartphones, computers, and tire pressure sensors. Besides electronics, they are also used in solar cells, which absorb photons from the sun and convert the energy into electricity.
Apart from semiconductors, silicon wafers are used in many different types of industries. These include the manufacture of ultrapure silicon for semiconductor manufacturing tools. It is also used in many other fields, such as electronic equipment. These applications are endless, and it is possible to discover some new uses for silicon. In addition to their countless uses, there are several other benefits. Aside from being used in manufacturing tools, silicon is used to produce parts for a range of other equipment.
Apart from semiconductors, silicon wafers are also used to develop chip and microchips. These are the electrical devices that command actions. Because of their stability, silicon wafers are used to build electronic appliances. Another important application of silicon is in calibration. A silicon wafer is a thin slice of silicon, and is used for a variety of applications. It can also be reused for other uses.
When it comes to making semiconductors, silicon is used in a variety of ways. A single silicon wafer is used to make a semiconductor, but the entire process of making a silicon chip is expensive. It's worth it, though, to have a pristine silicon wafer. It's worth the investment to create a quality product. So what are some of the most common applications of these microchips?
The first application of silicon wafers is in semiconductor manufacturing. The semiconductors are the building blocks of electronic products. These products are a combination of different components, and a silicon wafer can be used in a wide variety of applications. A transistor is made up of several layers. A silicon wafer is a thin sheet of silicon that has two layers. During the process of manufacturing, the two layers of silicon are bonded together. Then, these two layers are separated by a chemical-mechanical-planarization polishing.
The most common use of silicon wafers is in electronic devices. They are the basic material for electronic devices. A silicon wafer is an excellent example of this. Its crystalline structure is the most pure material ever created, and is perfect for most applications. The purity of a silicon crystal is important for this purpose. It's the basis of semiconductor technology. It's also used in other industries that depend on electronics.
The semiconductors used in these devices are the main element in modern electronics. They are the basis of all modern devices and are used in everything from smart phones to computers. While other semiconductor materials have been tested, silicon has proved to be the most reliable choice. It's also used in tires. As a result, these semiconductors are widely used in tire pressure sensors. They are also used in solar cells. Since they absorb light, they generate electricity.
How is a Silicon Wafer Made?
So, how is a silicon wafer made? It is first made by purifying silicon sand and then growing single crystal ingots. These single crystal ingots look like huge sausages. After the ingots grow to the proper diameter, they are ground into thin slabs for further processing. A diamond edge saw is then used to slice the resulting silicon wafers. This final step polishes the silicon wafer so that the edges are smooth.
Next, the silicon ingot is ground into a rough diameter. It is marked with a notch for orientation, and the surface is then sliced. The diamond edge saw is used to cut the wafers with the minimum possible damage. It also minimizes the amount of variation in the thickness of the resulting silicon wafers, as well as bow and warp defects. Once the ingot has been cut to the correct size, it is then checked for dimensional accuracy and sliced.
Once the silicon wafer is cut to the correct diameter, it is subjected to a chemical process called sputtering. This involves firing various elements into the silicon melt to modify its properties. It requires a clean room, and the technicians wear special suits. The resultant silicon chip will be used in PCs, electronic devices, and other products. So, how is a semiconductor made? This is an incredibly complex process and you can learn more about how it's made in a few simple steps.
The silicon wafer then undergoes a series of steps, including polishing, cleaning, and testing. Once a silicon wafer passes these steps, it is placed into a crucible. The next step involves the formation of a thin film of silicon above the seed. The silicon film adheres to the seed due to surface tension. As the seed is lifted up above the melted silicon, the atoms in the molten silicon attach themselves to the crystal structure of the seed.
The first step in the process of silicon wafer production is to grow a silicon ingot. This can take up to a month, depending on the quality and size of the silicon ingot. The CZ Method is the most common method of growing a silicon ingot, but there are also other methods, such as the Float Zone technique. Polycrystalline silicon pieces are placed into a quartz trough where they are left to grow.
Once a silicon ingot has been fully grown, it is then ground to a rough diameter. It is then cut into slices with a diamond edge saw. The diamond edge saw minimizes the amount of damage caused to the silicon ingot, and helps to produce a silicon wafer with the required thickness. In addition to the diamond-edged slicing, the diamond-edged saw is also useful for making smaller silicon wafers.
The next step of silicon fabrication is the growth of the seed. It is important to increase the size of the seed because it helps to increase the diameter of the silicon crystal. The increased diameter of the seed allows the growth process to continue. However, the seed is lifted above the silicon melt. The thin film is held together by the surface tension of the silicon. As the seeds grow, they begin to align with the crystal structures of the seed.
After the seed is placed on the silicon wafer, it is sputter-blasted. This process creates a thin film of silicon that adheres to the seed. The thin film is used to create the silicon chip. It is not a silicon wafer - it is a crystal. The seeds are placed on a quartz crystal that is shaped like a wafer. It is a crystalline structure.
After the silicon ingot is fully grown, it is polished. Then, it is examined under high intensity lights to determine its quality. The silicon ingots are then packed in vacuum-sealed plastic bags. This prevents moisture from affecting the silicon wafers during storage and transport. In the final stages of the process, the silicon wafers are polished to the right diameter. Then, the silicon ingots are then sliced, and the silicon ingots are ready for further processing.
Silicon Wafer Manufacturers
The market for silicon wafer is very competitive, as there are several different types and qualities of the material. UniversityWafer, Inc. bridges the fractured industry and is a leader in supplying silicon wafers in small and large quantities. In general, there are three main categories of silicon, and each of them has a different price. The highest quality is known as a prime wafer, and it meets high standards for cleanliness and flatness. The next most expensive type of silicon is called a test-wafer, and it closely matches the quality of a prime one. The lowest-quality type is referred to as a reclaimed, which is made by polishing patterned silicon.
The semiconductor industry in the United States is rapidly growing, with over 80 wafer fabrication plants located here. Companies that are growing in size include Apple, Broadcom, Qualcomm, and AMD. These fabless firms have expanded their operations and are driving demand for silicon wafers. The region has been a significant revenue contributor for semiconductor silicon-wafer manufacturers in recent years. However, it is likely that this trend will continue through the forecast period.
In the United States, five of the leading semiconductor firms have formed a consortium to pursue a transition to 450-mm wafers. The U.S.-based group is focused on the development of equipment that will process these 450-mm-wafers. The consumer electronics industry is driven by technological advancements, and this trend will continue to drive demand for silicon-wafers. It also means that consumers will have greater access to high-end products, and more money for the consumer.
Among the top semiconductor wafer manufacturers are SK Siltron Co. Ltd., Siltronic AG, Shin-Etsu Handotai, SUMCO Corporation, Globalwafers Co., and Applied Materials. The quality of a silicon-wafer is important to its suitability for any given application. The higher the quality, the more expensive it is. In addition to this, the price of a silicon-wafer is very sensitive to the quality of the material.
Although the memory market is recovering, the demand for silicon-wafers has continued to increase. Several companies are supplying silicon-wafers to the memory market, but there are still challenges in a number of other markets. The global semiconductor industry is competitive, and companies that can produce a single wafer are usually the best ones to choose. A good manufacturer will offer a range of materials to suit your needs and budget.
In addition to manufacturing a variety of silicon-wafer types, there are some companies that specialize in custom-designed silicon wafers. They may be able to customize these for you based on your specifications. Another way to find a silicon-wafer manufacturer is to search for a supplier that specializes in the specific type of material you need. A good manufacturer will provide the material you need. You can also search online to find the best prices on the Internet.
There are many different types of silicon wafers available. The main characteristics that define a silicon-wafer are its size and dopant. A semiconductor wafer with the highest die count will be more expensive than one with a smaller area. The cost of silicon-wafers is often dependent on their surface area. This increases the cost of the material, which is a crucial factor in semiconductor manufacturing. There are a few major companies that manufacture and supply semiconductor materials.
These companies are known for their reliability and expertise in silicon-wafer manufacturing. They are able to meet the strictest quality requirements for the material, making them ideal for semiconductors. The companies that specialize in silicon wafers should have a track record of quality and consistency. A high-quality product will be produced by a trusted company. The best companies are able to keep their customers happy, and if you are looking for silicon-wafer products, then you should look at their reputation.
The quality of silicon-wafers depends on their cleanliness. It is necessary for them to avoid particles that could cling to the surface of the wafers and disrupt the flow of electric currents. A clean room is the best place to clean and maintain these products. The cleaning process takes place in a clean room with specialized gears and machinery. In some cases, it can take days for the products to be finished.
Why is Silicon More Efficient Than Germanium?
The bandgap in silicon is responsible for its low sunlight-to-electrical energy efficiency. This barrier ![]() prevents the semiconductor from efficiently converting higher-energy photons like those found in blue, green, and yellow light waves into electricity. By contrast, silicon is much more effective at converting lower-energy photons, such as those present in red light waves. This is because the bandgap is larger in amorphous silicon, compared to its counterpart in germanium.
prevents the semiconductor from efficiently converting higher-energy photons like those found in blue, green, and yellow light waves into electricity. By contrast, silicon is much more effective at converting lower-energy photons, such as those present in red light waves. This is because the bandgap is larger in amorphous silicon, compared to its counterpart in germanium.
The difference in ICBO is due to the fact that the ICBO of silicon and germanium is ten to one. The ICBO of silicon will double at a given temperature while that of the former doubles at the same temperature. This difference is important for the efficiency of solar panels. The difference in ICBI is important, as it gives us a sense of how much power a material is capable of producing.
Although both materials have similar electrical properties, they are incredibly different in other ways. Silicon has a higher free electron count and has a lower ICBO than germanium. That means the Collector cut-off current in silicon is smaller and will vary less with temperature than that of the germanium counterpart. This is an important feature for many applications and will ensure that a device works at higher temperatures. And it's a win-win for all users.
Another difference between the two elements is the amount of energy they absorb. The former's high resistance to heat makes it an ideal candidate for high-voltage batteries. However, germanium has a higher threshold voltage. This is a major advantage for silicon diodes, but it can't compare with the latter. Therefore, silicon is more efficient than germanium when it comes to solar cells. You'll save money and have a more efficient system.
The two materials have the same electron density, but silicon has a lower lattice constant than germanium. Its electrical properties are identical, but the two materials differ in their lattice constants. Because of this difference, silicon is much more efficient in semiconductor applications. The ICBO value is the difference between the two materials. This is the most important factor in deciding which one is better.
The ICBO of silicon is a few times greater than that of the ICBO of germanium, and the ICBI in a germanium solar cell is twice as high. This difference is caused by the different thermal conductivity of the two materials. The resulting electric current is proportional to the energy in the solar panel. This difference between silicon and the other material is similar. They both require the same amount of energy to convert light to electricity.
Because silicon is more expensive than germanium, it is less expensive. In fact, it is far cheaper. The potential barrier of silicon is significantly higher. Both materials are cheaper to make than their counterparts. This makes them a better choice in semiconductor production. But they are not the only difference. The two materials are similar in their electrical resistance. The difference in their price is only in the amount of sunlight they can absorb.
As a result, they both exhibit similar characteristics. Both materials are relatively rare. This means that they are less expensive than silicon. Moreover, silicon is more durable and less expensive than germanium. If you want to make a semiconductor, you should choose a material with lower resistance. This is because it is easier to work with and maintain than germanium. It is also less likely to break. It is more expensive to process than other materials.
In addition to this, silicon has higher ICBO than germanium. While germanium is more expensive, it is also rare. It has a much smaller collector and is more efficient than germanium. Furthermore, Germanium is destroyed by high temperatures. This makes silicon the best material for semiconductor devices. The higher the ICBO, the more effective the semiconductor is. This is why silicon is better than the other material.
https://www.youtube.com/embed/YfyuzJXu0C0
How do Silicon Anodes Speed Up Electric Vehicle (EV) Charging
New anode technology that uses a thin film of porous pure silicon could lead to less-expensive lithium-ion batteries for electric vehicles that charge in just a few minutes and provide over 200 mile range. The technology could help increase an EV’s range by 30 percent or more.
Li15Si4 is the new material that combines silicon with lithium. UniversityWafer, Inc. can help researchers source the material for their lab.
There are many questions about the performance of electric vehicle batteries. The EV industry has a wide range of anodes to choose from, but all of them have one thing in common: they require a lithium-ion battery system. The majority of EV batteries contain graphite, but there are several types of anodes that use other materials, such as silicon, graphite, or carbon.
Silicon powders with particle sizes less than 200 nanometers have a high surface area, which enables them to![]() withstand fast-charging cycles. However, a silicon anode can develop swelling issues and can affect battery performance. This is a common problem among electric vehicle batteries, and the research is ongoing. But, in the meantime, designers are working with material scientists to develop advanced formulations and nanotechnologies.
withstand fast-charging cycles. However, a silicon anode can develop swelling issues and can affect battery performance. This is a common problem among electric vehicle batteries, and the research is ongoing. But, in the meantime, designers are working with material scientists to develop advanced formulations and nanotechnologies.
A recent study has identified a gap in the current industry-based lithium-ion battery technology. Graphite anodes have reached their limits in terms of power density, so manufacturers are turning to new materials to make their batteries more efficient. This is why silicon anodes are being developed in tandem with graphite. But despite these advances, there are still many challenges. The battery life will decrease significantly and the cost of production will increase.
While silicon is not the best material for an electric vehicle anode, it does have the potential to increase battery life and efficiency. Researchers from UC San Diego are developing an all-silicon solid-state battery that can offer long-term energy density while speeding up the charging process. But the downside of silicon anodes is that they tend to expand and degrade quickly, which means that they aren't suitable for commercial use.
While it is difficult to determine how silicon anodes will improve electric vehicle battery performance, the next generation of batteries with silicon anodes have the potential to charge EVs up to 80 percent of their capacity in just five to ten minutes. Unlike graphite, however, they have limitations in terms of their longevity. They also tend to expand and degrade when they are charged with liquid electrolytes.
The future of electric vehicles is a bright one. A new all-silicon solid-state battery is a step in the right direction. EVs have more energy than ever before, but their battery performance will depend on the quality of the silicon anode. The more energy dense the anode, the better the performance of the EV. But there are drawbacks to silicon anodes.
Currently, the most successful electric vehicle batteries are made of graphite. These are very efficient, but they are limited by the density of silicon. Graphite anodes are very bulky and don't charge EVs quickly. It takes a long time for an EV battery to fully charge. That's why many EVs will need a fast-charging network.
The main benefit of silicon anodes is their energy density. When silicon is added to an EV battery, the energy density will double. The increased power output will also boost battery life. EVs that are built with lithium-ion batteries will be more expensive than those made with other materials. Therefore, it's essential that EVs use the fastest-charging technology. So, how do Silicon Anodes Work?
UC San Diego researchers have developed a solid-state battery with an all-silicon anode. The all-silicon anode ![]() is designed to provide long-term energy density and fast charging. Compared to graphite anodes, silicon has a much higher energy density than graphite. Although there are several disadvantages to silicon, it's worth noting that the energy density of lithium-ion batteries can increase by up to 20%, while the cost of EVs can be reduced by half.
is designed to provide long-term energy density and fast charging. Compared to graphite anodes, silicon has a much higher energy density than graphite. Although there are several disadvantages to silicon, it's worth noting that the energy density of lithium-ion batteries can increase by up to 20%, while the cost of EVs can be reduced by half.
The anode's thickness limits its ability to charge fast. This means that the ions must travel further through the anode's twisted paths. These are not only unattractive, but can also kill the battery's performance. These problems are only exacerbated by thin anodes, so EV manufacturers need to be able to make the most efficient anode possible.
Silicon The Element Defined in Detail
However, the latest results confuse what we know about the element and the individual elements on its surface. To be sure, researchers should know all about silicon by now, but they don't, at least not yet.
Silicon was first identified in 1824 by Swedish chemist Jons Jacob Berzelius, but it has been worshipped by a number of other chemists and physicists over the last two centuries, from the late 19th century to the early 20th century.
Interest in silicon increased in the late 1970s and early 1980s, when silicon transistors were developed to replace vacuum tubes in electronic devices such as computers, televisions, and mobile phones. It has since become the preferred material for electronic devices because it can make small circuits and integrate them into small chips.
Silicon ushered in the so-called silicon revolution, which has changed society and permeated every corner of daily life. When we speak of semiconductor technology, we are talking about silicon crystals, which are normally cut from larger crystals to form thin wafers.
This has enabled enormous computing capacity, which has reshaped the world by processing huge amounts of data and continuously accessing valuable information. While crystalline silicon has long been studied, the surface of the thin silicon layer has played an important role in the development of computer chips, as it is a key component in many of its applications. There is no doubt that the basic properties of the silicon surface are still unknown and widely discussed.
He joined IBM's Thomas J. Watson Laboratory to help develop and apply new surface inspection techniques. PhD student, has been working with metal surfaces since his doctorate and continues to work well with them and understand them well, as well as facilitating the development of new techniques.
At the time, I was an outsider in silicon surface research, so Mr. Cary asked me why I wasn't interested in silicon surfaces.
When the opportunity came up to do a new kind of measurement that no one had done before, I saw an opportunity and thought, "Why not?
The new attempt to study silicon surfaces involves understanding Si (111), which has been widely studied since 1957 but whose surface structure has never been understood. refers to the fact that the crystal is halved and a flat plane of atoms remains on the surface. To measure this, a surface must be cleaned and heated to remove dirt, with its atoms arranged like marbles in different configurations.
The annealed Si (111) surfaces exhibit a diffraction pattern of 7x7, which is derived from the unusual atomic structure they possess. This pattern fascinates everyone who looks at it, and it has undoubtedly become one of the most widely studied semiconductor surfaces, if one excludes none. The latest discovery, which will be discussed later in this article, is based on initial studies of Si11 surfaces.
The new temperature-dependent measurements of 7x7 show many interesting electronic transitions that were not observed before. Normally, if a surface is a semiconductor, it would be expected to become an insulator at low temperatures, but more importantly, it would be insulated at lower temperatures (about 50 K). In 1983, a theoretical model of the 2x1 structure was proposed and established, but the structure and chemical composition of a 7X7 surface was much more complex and elusive. In the 1980s, a new method of studying silicon surfaces - the Si (111) diffraction pattern - was developed, which allowed us to study other properties of this pattern. What people knew at the time was that if you broke a crystalline silicon rod in 111 directions, you would get a simple diffraction pattern of 2X1, and if the 2X2 surface were heated, the surface would form the 7Z7 pattern and be very stable at high temperatures.
In general, such behaviour has a specific temperature dependence, but in 7x7 we found another temperature dependency. The surface is neither semiconducting nor metallic, so it is a very unusual effect to create electrons on the surface of the metal isolate, depending on how the electrons are aligned.
This was proposed in 1985 to accommodate diffraction experiments, but the problem was that the calculated structure was always metallic, which contradicted the experiments. The 1985 7x7 structure, which was confirmed as the lowest energy and most stable structure, was revealed in the 1990s, when calculations were mature and could be performed to predict the complex structures of the 7X7 surface.
This became the unsolved paradox of the silicon surface and the subject of a so-called scanning tunnelling microscope, for which he and other IBM colleagues received the Nobel Prize in Physics in Zurich in 1986. The paradoxes of 7x7 were rediscovered in the 1990s, this time by Bob Kowalski and colleagues at IBM, using a new device designed to perform electron spectroscopy on silicon surfaces at atomic resolution.
The high stability of the STM design made it possible to see the electron clouds in different places on different surfaces and atoms and to dissolve their energy into atomic solvents. However, the theory did not predict the surface conditions observed at atomic resolution in 1983 and 1986. Initially, experimental measurements and their interpretation were a valid form of simplified calculations. Several researchers confirmed the new electronic state at the time, but again, no one had a clear explanation.
In the insulation of floors, the paradox of the 7x7 surface became the basis for the development of a new type of high-temperature, low-energy electronic state of silicon.
I left the lab in 1993 to pursue other interests and retired in 2005, completely in the paradise of my surroundings in Florida. I # ve never played so many rounds of golf in a year, caught so many fish in a single day, or played and played so long, at a time when the game of golf seemed to be getting worse, not better.
That's when I decided to write to my grandchildren about why I became a scientist and what it means to be a scientist. Even then, I remembered all that and was kind of confused about what I was ever going to be.
After two years of studying the results of the past and consulting the literature, I discovered two also more recent paradoxes and why they arose. To my surprise, despite many new studies, they have never been resolved, and there are many structures proposed over the years that would not fit either. These discoveries were made by attempting a reverse engineering process, taking into account certain features that an alternative structure might take into account. They are all based on many experiments, which today tell us much more than theoretical calculations and the state of the art.
To my surprise, I found a new structure that takes into account these unusual paradoxes, but not in the same way as the previous ones.
The trick is that in a very complex system, there can be different arrangements of atoms that look like structures from one angle but are connected by icicles stacked upright on a tray. When you look at it from the side, you see that you are actually standing on the cone, and when you look down, it is like a ball. At close range, each rung can have a different shape, such as a triangle, a circle or a cone with different shapes and sizes.
The original structure in 1985 was proposed as a two-dimensional (2D) structure, similar to that in the atom, but the details of the new structure gave it distinctly different properties. The electrons behave very differently when they are in this new 2d frame, and there are now bonds. In the 2000s, everyone in the scientific community still believed that the original 1985 structure was correct. Now, however, it has been proposed again, this time with a different structure.
In 2008, many of the researchers working on the surface switched to studying graphene, which is best known for its use as a surface for the production of high-performance electronics. Graphene is one of two materials based on carbon, but whose atoms are arranged in a hexagonal structure.
As a result, graphene has a number of properties, the most striking being a very high electron mobility, which is important for electrical devices. The discovery of graphene was awarded the Nobel Prize in Physics in 2010 for its role in the development of high-performance electronics and its use in materials science.
For some time now, there have been efforts to adapt other 2D structures for electrical devices. However, graphene formation on substrates has proved problematic as its formation in the substrate is crucial for highly integrated applications such as electronic devices and electronic components.
The role of silver surfaces is called into question, however, as the 2D character of silicon atoms in silver must be preserved, especially as the silicon layers become thicker. Researchers at the University of California, San Diego School of Engineering have discovered in a promising new electronic material that silicon can be used to form a 2d structure similar to graphene. They succeeded in this by cultivating a monolayer of silicon on a silver surface. The monolayer of 2D silicon grown on silver has several properties that correspond to those of graphene, such as a high surface area and strong electrical conductivity, which silicon requires as an ideal material for use in electronic devices and electronic components.
What Silicon Wafers are Used for Infrared (IR) Imaging?
Scientist Requests: I am looking for silicon wafers for IR imaging. They should have a diameter of 40mm and a thickness of 0.5-1.5mm.
I would need a small quantity (~20). What other specs do you refer to? We need them to be as transparent as possible in the IR range (~9um) for thermal imaging.
UniversityWafer, Inc. Quoted:
Silicon wafers for IR imaging. diameter of 40mm and a thickness of 1.5mm,double sides polished,quantity (~20). as transparent as possible in the IR range (~9um) for thermal imaging.
Please contact us for pricing and Reference 265512
Inspecting Silicon Wafers for Infrared Imaging
Short Wave Infrared (SWIR) Cameras can be used to inspect and monitor silicon wafers to detect ![]() defects that could affect the end product's performance. In semiconductor manufacturing, the alignment of a silicon wafer is vital to ensure the correct functioning of the device. SWIR Cameras can view the internal and front surface structure of a silicon photonic crystal, which is the basic material for photonic crystals.
defects that could affect the end product's performance. In semiconductor manufacturing, the alignment of a silicon wafer is vital to ensure the correct functioning of the device. SWIR Cameras can view the internal and front surface structure of a silicon photonic crystal, which is the basic material for photonic crystals.
Infrared light is the most effective wavelength, able to penetrate objects that are invisible to optical telescopes. This energy is also useful in sensing, as every object on Earth emits heat and can be detected by IR sensors. Infrared imaging is a critical application for this technology. To understand how this technology works, we must first understand what IR is and how it works.
IR rays cause deep heating in tissues, and this causes the heart to expand. The increased volume of the heart increases the blood flow, and increased circulation results in accelerated healing. This technology is becoming a vital part of x-ray imaging for medical devices. Infrared images have a wide range of applications. One such application is in a medical facility, where the medical team can easily diagnose and treat patients with high-definition imaging.
The IR wavelengths produced by silicon wafers can be as long as 900 nm. Infrared pictures are useful in mapping ocean currents and eddies. These images are also used to measure carbon black content in ink. Without an infrared-sensitive imaging system, the infrared range of the spectrum will be far less sensitive than the visible range.
What Wafers are Used for: Merging Bottom-Up with Top-Down: Continuous Lamellar Networks and Block Copolymer Lithography?
Scientists requested:
I would like to ask your opinion...what is the most appropiate silicon wafer type
for Block copolymers. For examble, polymner semicrystallized segments like
Polyethyelene, Polyethylenoxide and other polar blocks like PMMA, Polypropylene and Polystyrene.Deposition method is for my case spin-coating
process. Thank you in advance.
UniversityWafer, Inc. Replied:
Were you trying to deposit copolymers on top of silicon and study the nucleation and/or crystallization? If so, we recommend the surface of silicon to be polished, orientation can be flexible, as for size, some research teams even dice the wafers to do similar experiments, therefore 1" or 2" wafers should be big enough for the purpose. Please reference #211777 for specs and pricing.
What is in a Silicon Wafer?
Silicon is the most abundant element in the universe, but it is the least efficient semiconductor, so the process of creating silicon wafers is a great way to cut costs and produce electronics in mass quantities. Because of this, the manufacturing of semiconductors from silicon wafers has been booming in recent years. This article will explain what is in a silicon based semiconductor and why it is used in electronics. You may also be curious about how silicon is fabricated.
Silicon is the most purest material ever made, which makes it a valuable component of an integrated circuit. An ordinary silicon wafer has few defects and is so pure that a diamond jewel looks dirty. The surface of a silicon wafer is smooth and flat, improving its purity and suitability for semiconductor devices. The two most common techniques of making silicon based chips are the Czochralski method and the Vertical Bridgeman pulling process. The Float Zone fabrication method is increasingly used because of its high purity and fewer defects.
A silicon based semiconductor is one of the purest materials ever created. A freshly-sliced silicon based wafer is so clean and smooth, it makes a diamond jewel look filthy. Moreover, silicon is also transparent to infrared light, which means that it can be used as a protective window for thermal cameras. The resulting mirror-like surface makes it a perfect material for creating a variety of high-tech electronics.
A silicon wafer is the main ingredient of integrated circuits. It is a composite of electronic elements. In fact, the silicon is the key platform of modern semiconductor gadgets, including computers, smartphones, cell phones, and tire pressure sensor systems. What is in a silicon wafer? This article will help you understand how a silicon wafer works and why it is so important to the manufacturing process. Once you understand the importance of silicon, you will be able to create better and more reliable semiconductor products.
A silicon wafer can be divided into several types, depending on its purpose. Generally, the first type is used to manufacture IC's, while the second one is used for production. These silicon wafers are used to create many different types of electronic products, such as cellular phones and semiconductors. This is why it is essential to know what is in a crystalline silicon chip. It will help you design smarter electronics.
The second type of silicon wafer is used to manufacture chips. These chips are very fragile and must be made of the best materials possible. Hence, it is important to know what is in a silicon wafer and its function. The first type is a semiconductor. The second one is a semiconductor. These two types of chips are created using a silicon wafer. The latter has the highest quality of any other semiconductor in the market.
The next type of silicon wafer is the Okmetic silicon wafer. It has a mirror finish. The last type is a non-crystalline semiconductor. A polysilicon wafer has no buried layer, so it can be used to make CMOS and bipolar transistors. This is a premium grade silicon. Unlike its lower-quality counterparts, it has no traces of metal.
The first type of silicon wafer is the Czochralski silicon wafer. This type of silicon wafer is the most common form of the material. It is also the most widely used in the technology industry. Its high purity makes it ideal for making electronic components. But what is in a silicon wafer? It is a thin slice of silicon crystal. The other kind of silicon wafer is the Vertical Bridgeman pulling method. It is the third most popular.
The silicon wafer is the most common semiconductor. It is used in the manufacturing of integrated circuits, which are electronic components that work together. Each integrated circuit is composed of millions of transistors, resistors, and capacitors. As a result, silicon wafers are necessary for electronic equipment to function. However, the underlying materials are crucial to the manufacture of these devices. The simplest of these are the simplest and the most efficient.
How Do Silicon Wafers Work?
If you've ever wondered how a computer chip is made, you've probably heard of silicon wafers. But what's all the fuss about? This article will give you the scoop. First, let's discuss what a silicon wafer is and how it works. A wafer is a flat disc, similar to a marble or stone, and a semiconductor is a piece of silicon that is a solid, but in liquid form.
![]()
Integrated circuits are made from silicon, which is the second most abundant element on earth. These devices contain hundreds or billions of tiny components, and even the smallest dirt spec can cause havoc. This is why semiconductors are manufactured in sterile environments. Clean rooms are filtered, and workers wear protective clothing. Pure silicon crystals are sliced into long, thin cylinders called ingots. These wafers are then cut into numerous chips.
The process of manufacturing an integrated circuit starts with a big single crystal of silicon. This crystal is then sliced into thin discs called wafers. Each of these discs is marked into dozens or hundreds of identical square or rectangular areas. After these are marked, a process called sputtering is used to coat different parts of the silicon wafer with various materials. This process gives the chip its unique structure.
The process of manufacturing an integrated circuit is complex. It contains hundreds of millions or even billions of tiny components. A small speck of dirt can wreck havoc at the microscopic scale. To keep everything clean, semiconductor workers work in clean rooms. These rooms are made of ultra-pure silicon crystals that are melted and cut into thin wafers. The chips are then sliced and assembled together.
To make a silicon chip, the silicon ingot is sliced using diamond saw blades. After being checked for purity, the silicon ingot goes through wire cutting, which creates a "kerf" on the surface of the silicon wafer. Fortunately, some firms are working on ways to make chips without a kerf, which would save them a great deal of money. If you're wondering how a semiconductor chip is made, you'll be happy to know that there are many steps to take, and there's nothing to worry about.
Once a silicon wafer is sliced, it undergoes a series of processes to create a single crystal. A newly sliced silicon wafer must be mirror-like and have a pristine surface. During the polishing process, it's held in a vacuum carrier. Specially designed pads are used to remove minuscule layers of the silicon wafer. Once a thin layer of silicon is formed, the resulting crystal is smooth and mirror-like.
A silicon wafer is a very thin, mirror-like disk with an optical and electrical property. A silicon wafer is the flattest object in the world. It is free of impurities and micro-particles. These properties make it the perfect substrate for a modern semiconductor. A silicon wax wafer is manufactured by several processes. The main step is to grow a single crystal of pure silicone, then cut it into thin wafers. Then, it's sliced into multiple chips.
The process for making semiconductors begins with the extraction of silicon from silica sand. This sand contains too much oxygen to be silicon. The sand is then mixed with carbon, which is then heated to 2000 degrees Celsius. The heat separates the silicon from the impurities and leaves a 99% pure product. After the sand is sliced into thin wafers, the silicon is ready to be used in electronics.
A silicon wafer is thinner than a human hair. The material can be smooth, glass-like, or rough. It is the most common material for semiconductors. A silicon wafer can be as thin as a grain of rice. A silicon wafer is a thin sheet of silicon that can be a few inches thick. They are thinner than the thickness of a human hair. A silicon wafer is a very thin sheet of the same material as a marble, but are very different.
A silicon wafer is a thin, glossy slice of silicon. A silicon wafer can be either round or flat. Some silicon wafers are made of plastic. Other types are made of metal. The process of making a silicon-based semiconductor wafer is more complicated. It starts by spinning molten silica in a crucible. Then, a seed crystal is inserted into the molten material. Then, it is slowly removed until a large, high-purity crystal forms. Finally, it is solidified into a rod. This process is known as the Czochralski or Float Zone.
Where Can You Buy Silicon Wafer?
UniversityWafer, Inc. sell Silicon wafers that can be used for a variety of semiconductor and electronic purposes. Versitile silicon has multiple uses and is excellent ![]() for many device applications. It is high in thermal conductivity and mechanical properties, making it perfect for high-temperature and MEMS applications. It also offers low power consumption and excellent performance. It is commonly used in the production of semiconductors, computer chips, optical components, photovoltaic cells, and solar cells. It is also used in the manufacturing of other types of materials.
for many device applications. It is high in thermal conductivity and mechanical properties, making it perfect for high-temperature and MEMS applications. It also offers low power consumption and excellent performance. It is commonly used in the production of semiconductors, computer chips, optical components, photovoltaic cells, and solar cells. It is also used in the manufacturing of other types of materials.
When it comes to purchasing this product, there are several factors to consider. First of all, it is very convenient. In the past, buying a silicon wafer from a manufacturer was difficult. Today, you can purchase a silicon based wafer from a reputable manufacturer. Moreover, the price is more reasonable. Most of the suppliers offer their silicon based products at a lower price. Second, they are very efficient.
The best way to buy a silicon wafer is to look for a supplier that has both long and short term business opportunities. Some of the companies sell raw material as well as finished products. Another way to buy silicon is through online auctions. The first place to look is a local store that offers semiconductor materials. These stores have a great selection of silicon based products. They will also have a variety of other products.
Another option is to purchase a silicon based silicon wafer. This is a practical way to buy a semiconductor since the parts are manufactured in large quantities. They can also be purchased online, which saves money and time for the customer. You can also choose a silicon wafer based on how much you want to pay for it. When you buy a silicon based wafer, you can find a good price.
Where can you buy silicon based on its purity? A good place to get a wafer that's more than a few hundred dollars is UniversityWafer, Inc. Located in South Boston, MA, USA innovation district. UniversityWafer, Inc. is one of the most friendly customer service and is often ranked as one of the best suppliers of semiconductor silicon wafers and services. Oursilicon wafers are sold to almost ever university and semiconductor related companies. If you need a silicon wafers then UniversityWafer, Inc. is great for one stop shopping.
Silicon wafers are thin disks of pure crystallized silicon that act as substrates for semiconductor devices. Compared to metallic substances, they are more stable and are widely available. Because they are widely available in nature, it is also easier to purchase silicon than other types of semiconductor. You can also make your own wafers. UniversityWafer, Inc.'s other products include Germanium (Ge), and Gallium Arsenide (GaAs).
Buying a silicon wafer is an excellent option if you need a larger quantity. They're available in various diameters and can be used for many applications. If you're looking to buy a silicon wafer for the manufacturing of solar cells, you can choose between a 6" ultra flat silicon or a 4" ultra-flat silicon wafer. It's also possible to find a larger size of a silicon wafer to suit your needs.
Why is a Silicon Semiconductor Used in a Thermistor?
If you've ever wondered why a thermistor is so important, then you've come to the right place. In this article, we'll talk about the various types of thermistors, the properties they possess, and the applications for which they're used. Read on to learn more. Here's a look at why a silicon semiconductor is used in a thermistor.
Using a Silicon Semiconductor in a thermistor
In a thermistor, the resistance of a semiconductor is proportional to the temperature. This resistance varies from positive to negative. In general, a thermistor has a negative temperature coefficient of resistance. Typically, the resistance of a thermistor is much higher than its ambient temperature, or T0, but a thermistor with a positive temperature coefficient of resistance is also possible.
One type of silicon thermistor is a spreading-resistance device. It is characterized by a silicon body and a layer of insulation. These metal contacts form ohmic contacts with impurity regions. Secondary contact means connect the silicon carbide semiconductor body to the electrical circuit, preferably by a braze or mechanical bias. This type of device is capable of sustaining high temperatures.
While this material is suitable for use in thermistors, there are several limitations to its use in these devices. Unlike bulk-type thermistors, which are made of two layers of silicon, semiconductor thermistors are generally low-cost and rugged. They respond quickly to temperature changes and are designed for low-temperature measurements. Various semiconductor materials can also be used for thermistors.
Theoretical considerations should be incorporated into the design process. The semiconductor should have a heat capacity Ce. The heat capacity of a semiconductor is dependent on the valence-band electron configuration. Using a silicon semiconductor in a thermistor has several important implications that go beyond the R(T,E) relation. For example, the electrical conductivity of a thermistor must be determined to be proportional to the heat capacity Ce.
Depending on the application, the choice between Si and Ge is usually a matter of reproducibility, ease of fabrication, and temperature sensitivity. Nevertheless, the latter material is a less preferred choice for sensitive thermometers, despite its low sensitivity. The effect of heat capacity on thermometer performance decreases with increasing power density, due to electric field effects and hot electrons. A rough figure of merit would be the sensitivity of the semiconductor under a certain magnetic field.
Various shapes can be achieved by anisotropic etching. Dry and wet processes can be used to customize the shape of the thermometer. A high selectivity mask is required for a wet process. Moreover, the etching rate of Si is important since a single layer of silicon can have different shapes. Consequently, wet etching of the silicon semiconductor can be used to produce both anisotropic and isotropic structures.
PTC thermistors increase their resistance when temperatures exceed Curie temperature. This property is advantageous because it reduces the probability of a thermal overcurrent situation. Moreover, it can also protect electrical systems from overcurrent situations by acting as self-resetting fuses. A PTC thermistor has several key terms and performance specifications. If you're looking for a reliable thermistor, consider using a PTC.
What are the Properties of a Thermistor
Thermistors are electronic devices used to measure temperature. The temperature-dependent resistance of a thermistor is known as its zero-load resistance (R0). At low power levels, R0 is about 25oC. At the same temperature, T is the resistance. Another property of a thermistor is its dissipation constant (k), which measures the power required to change the resistance by one degree. The k value of a thermistor is non-linear, but a linear relationship can be assumed for small temperature changes.
The temperature is measured through the impedance response across the PN junction of the thermistor. A silicon carbide body is used to sense temperature. As the temperature increases, the impedance changes. The low bias voltage is found to be a logarithmically proportional to the temperature within the temperature range between -200degC and 1,000degC. This nonlinearity enables external processing to measure temperature.
A negative TCR thermistor, as its name suggests, decreases in resistance as the temperature increases. The characteristic curve of a negative TCR thermistor shows that resistance decreases with temperature. This property is often found in component data. Resistors are commonly used in many different applications. However, their resistance changes in a small way with large temperature changes. In contrast, semiconductors provide larger variations for temperature changes than resistors.
A high impedance junction thermistor has a PN junction that allows it to sense temperatures from -200degC to more than 1,400degC. A silicon carbide body comprises a silicon carbide body and a second impurity region, with the PN junction separating these two regions. A metal contact, preferably tungsten, is bonded to the housing via diffusion bonding to the silicon carbide body.
A semiconductor thermometer can be used in bolometers and calorimeters. For a bolometer or calorimeter to work, the sensitivity of the thermistor must be sufficiently large. A thermistor can be tuned to a large range of temperature by carefully selecting the amplification of internal thermodynamic fluctuations. In a calorimeter, the amplification is much smaller than the signal's size.
The properties of a silicon semiconductor used in a thermometer include the thermal resistance, the thermal conductivity, and the doping density. The difference between these properties depends on the temperature and doping density. At room temperature, a silicon semiconductor can be easily ionized, while a Ge semiconductor is rapidly ionized at low temperatures. Neither material has good reproducibility.
The electronic heat capacity of a silicon semiconductor used in a thermometer is a flat temperature dependence. It is constant below 0.1 K and steeper at higher temperatures. At 0.2 K, the electronic heat capacity approaches g=1. The value is in qualitative agreement with the other measurements, but a slight shift in the absolute value could be a result of compensation. The temperature dependence of the electronic heat capacity changes when doping density increases.
Applications of thermistors
Thermistors are electronic devices that measure temperature by measuring resistance. The range of temperature is relatively small, and thermistors are often used in cell phones. Thermistors are inexpensive and can be smaller than SC-70 packaged analog temperature sensors. The main problem with thermistors is their accuracy. Because the resistance of a thermistor decreases quickly as the temperature rises, a high-resolution ADC is required to measure the temperature accurately.
Typical silicon-based thermistors have linear behavior and minimize the processing burden associated with Steinhart-Hart equation. A fourth-order polynomial regression formula is used for calibration. The formula includes a temperature-dependent constant, T, and the calculated resistance value, R. The polynomial coefficients A (0-4) determine the temperature range of the device. A fifth-order polynomial regression formula is also available, reducing the processing burden.
Thermocouple thermistors are often used in power supply circuits. Their low-resistance characteristics allow higher currents to flow during normal operation. NTC thermistors are typically larger than measuring type thermistors, but they are specifically designed for in-rush protection applications. Despite their negative characteristics, NTC thermistors offer a range of benefits, including better accuracy.
Another way to improve thermistors is by improving their reproducibility. By implanting boron dopants on a silicon layer, it is possible to lower the 1/f noise parameter by a factor of six. Moreover, a boron-doped silicon layer has high reproducibility. A boron-doped silicon device would have better reproducibility, which means lower energy costs.
Thermocouples are important in many different applications. They provide an indication of a temperature when they are inserted into a thermal sensor. This thermistor has an extremely low resistance, which is one of the biggest reasons it is used in the electronics industry. In addition, silicon thermistors are relatively inexpensive compared to their counterparts, and have a positive temperature coefficient. Therefore, silicon thermistors are widely used in electronics.
Temperature-sensitivity is important to determining the sensitivity of a thermisor. In addition to resisting heat, thermistors are sensitive to electrical currents. Depending on the application, the resistance will change with the temperature. However, there are some exceptions to this rule. Generally, a thermistor's sensitivity is dependent on both temperature and its composition.
In addition to being used for temperature measurement, NTC thermistors have several other uses. One of the most common uses is in the construction of circuits requiring temperature compensation. In addition to measuring temperature, these devices can function as liquid sensors, or current limiters for inrush current. Their dissipation constant is changed by the presence of liquid. If a sensor needs to monitor low temperature, it can be used in a thermal switch.
Although NTC and PTC thermistors are widely used, the benefits of a silicone sensor are more diverse. NTC thermistors have been widely used in medical and automation applications, while KTY thermistors have been used in electrical machines. In addition, KTY thermistors are ideal for narrow copper windings in low voltage motors. If a thermistors can be used in both areas, they can be useful for the process monitoring and the result.
Does DNA Have a Silicon Analog?
Does DNA have a silicon analog? Probably not. DNA has a wider range of calculations than conventional computers can handle, but it also requires fewer DNA strands. In fact, it would probably be easier to construct an analog circuit than a digital one. Here are some of the main reasons why analog DNA is more efficient. Let's explore some of them. And don't forget to check out our previous articles on this subject!
![]()
Base pairing
It is important to understand how base pairing in DNA works. Accurate pairing is crucial at multiple points in the transmission of genetic information. With the growing complement of DNA analogs, evaluating their mechanism will be essential. Electrostatic and steric effects can influence base pairing, with their relative importance varying according to context. Proteins can also modulate the affinity and specificity of bases, and different proteins have different mechanisms for accomplishing these tasks.
Metal-mediated base pairs represent a prominent area of research. These structures connect complementary nucleobases by hydrogen and coordinate bonds to an embedded metal ion. The use of these bases enables the site-specific introduction of metal functionality into nucleic acids, making them ideal for use in DNA nanotechnology. The main objectives of this minireview are to outline the basic requirements for metal-mediated base pairs and to discuss their design from an experimental as well as conceptual perspective.
As DNA polymerases have recently developed nonpolar adenine and thymine analogs, they can replicate with high efficiency. The T7 DNA polymerase and the Klenow enzyme both contribute to base pairing. Despite the small positive role played by the enzyme, steric effects are also significant. Moreover, many laboratories have demonstrated the synthesis of DNA base pairs without hydrogen bonds using multiple designed bases. Interestingly, Hirao's most successful pairs take advantage of a minor groove hydrogen bond acceptor.
Toehold replacement
In an effort to simulate the function of the toehold of a biological molecule, Li and colleagues developed a DNA computer that can read and write the data on the strand. Although the exact output of the DNA computer is unknown, more blanks are filled as the project moves closer to completion. The team's strategy mimics the allosteric regulation of the activities of enzymes found in nature.
Toehold replacement in DNA is accomplished by binding an input single stranded DNA sequence to a partial double stranded sequence, which is partially complementary. When the two strands are complementary to each other, the toehold can reversibly interact with the gate, which displaces the partially double stranded sequence. The toehold is therefore a reversible process.
Toehold-mediated strand displacement is an important aspect of DNA information processing. It depends on three key mechanisms: automating synthesis and Watson-Crick base pairing. In addition, toehold-mediated strand displacement has been found to be crucial for the development of DNA circuits. Using a modular construction method and digital logic, these techniques have made it possible to build complex DNA circuits. In the latter case, a signal strand may induce or trigger strand migration.
Short oligonucleotides
Oligonucleotides are short DNA or RNA molecules with wide applications. Synthetic and cell-made DNA are both similar in their structural and chemical properties. They are also crucial for polymerase chain reaction (PCR), which is a biotechnology technique that replicates DNA by copying a strand one time at a time. These molecules are formed by using a synthesis process called solid-phase chemistry.
The nano-porous ceramic chip, DNAReax, provides up to 100 times more high-quality oligos per square millimeter than a silicon chip. These DNA microarray chips are turn-key replacements for silicon chips, with a hundred times the available surface area. Ceramic chips have controllable spacing between individual DNA chains, eliminating errors caused by overcrowding on silicon chips.
The synthesis of a short oligonucleotide DNA array is possible through a process that utilizes selective nucleases. The nucleases are triggered to cleave at specific locations and release a sequence for triggering downstream logic gates. These structures are then stored on a silicon nanowire, which can then be used to build complex electronic devices.
As the oligonucleotide length is essentially unlimited, the synthesis time is proportional to the sequence length. Fig. 2 shows how the length of a short oligonucleotide DNA is equivalent to a silicon nanowire. The sequence shown here was synthesized in 3 h. This process is not limited to RNA; short oligonucleotide DNA sequences can be made as long as 200 nucleotides.
Positive-feedback loop
The mechanism by which cells regulate their own DNA has a positive-feedback loop. The cyclins in the G1 region are responsible for triggering this system. When they are expressed, they advance their own expression. However, during the cell's division, the cyclins are inactivated. This mechanism ensures that the cell will finish its life cycle. Molecular simulations of cells show that the mechanism is similar to the one found in living organisms.
Molecular biology has long studied the effects of positive feedback on the environment. In a feedback loop, a small disturbance will amplify the effects of the initial perturbation. For example, when the head of a fetus pushes against the cervix, it sends a nerve impulse to the brain, which then signals the pituitary gland to secrete a hormone called oxytocin. Oxytocin travels to the uterus, where it causes contractions and pushes the fetus closer to the cervix. The process of childbirth then takes place.
Another example of positive feedback is the network effect, where more people join a network, and the network expands more quickly. Viral videos, for example, are examples of positive feedback. Initially, a video goes viral, and then more people see it and republish the link. In some situations, this can lead to a positive feedback loop, but it also limits its effects. The population size of a population determines how much feedback can be produced.
DNA logic gates
The DNA-supramolecule prototype has high flexibility, reconfigurability, and parallelism, and it may have a promising future in multiplex chemical analysis and molecular computing. Several research teams are pursuing this concept to develop a silicon analog. A DNA-supramolecule prototype is currently being fabricated at the University of Washington. Its reversible strand displacement mechanism enables efficient design and operation in a large circuit.
DNA-based logic gates are important modules for biosensing and molecular computing. They have the potential to provide a wide range of logical operations, and they can be incorporated into complex structures with multiple copies. In this study, a DNA-based logic gate complex is constructed and analyzed to show how its fluorescent output can be programmed. This work is an important step in the development of DNA-based computers.
In addition to their silicon analog, DNA-based logic gates can perform aptamer-based screening for more cells. These aptamers are available against many cell types, allowing for high-order multiple cell-surface marker identifications. As the number of aptamers continues to grow, DNA logic gates may become increasingly useful for cancer-treatment and detection. In addition, they can also help detect cancer-related biomarkers.
To understand how the two types of duplexes work, one must first understand how the two-stranded DNA interact. Interestingly, they are complementary, resulting in a similar effect. D2 aggregates with D1 and reduces the fluorescence of the single-stranded D1.
Applications of DNA barcoding
DNA barcoding can be used to identify single species, communities of species, and cryptic diversity information. DNA barcoding can also help with the identification of illegally taken wildlife and products derived from it. In the United Kingdom, for example, DNA barcoding has been used by mycology groups to identify species of fungi and mushrooms in the UK. These efforts are part of the Kew's Lost and Found Fungi project.
Plant DNA barcoding has been applied to species inventories, but the discriminatory power of DNA barcodes for plants is less than that of insects. In many cases, morphological data is not enough to identify species, and DNA barcoding can facilitate species-specific genetic identification of belowground roots. Kesanakurti et al. (2011) investigated the spatial distribution of plant root diversity. The findings revealed that the species-level DNA barcoding can accurately identify sterile and juvenile root samples.
DNA barcode projects use samples obtained from various sources, including private collections of amateur taxonomists, museum specimens, and citizen scientists. The museum specimens are carefully screened for DNA quality. The DNA barcodes obtained from citizen scientists are compared to known sequences. This ensures that the DNA barcodes generated from unknown samples are the correct ones. They are also useful for tracking and preserving species for future study.
Video: Learn about DNA Editing
What Material Will Replace Silicon?
Silicon, the wonder material that has powered our tech industry for the last seventy years, is nearing its end. This element can be shaped into transistors and act as both a conductor and insulator. It is essential to the entire digital revolution, from COVID-19 vaccines to TikTok. As such, the tech industry is looking for alternatives. What will replace silicon? Here are a few possibilities.
Graphene
The future of the semiconductor industry is uncertain. Silicon has been the mainstay of electronics for over 50 years, but there are several new materials vying for supremacy. Graphene is a promising candidate to replace silicon, and the use of the material will enable manufacturers to create more efficient semiconductor chips that last longer. While it is not ready to replace silicon altogether, it is already being used in many niche applications.
The use of graphene as a semiconductor chip is in the early stages, but the potential is enormous. Graphene is ideal for memory applications, but it is not a suitable candidate for transistors. Transistors need a band gap that allows them to switch on and off. It is also difficult to grow graphene at high-density levels, so graphene is not suitable for this task. Bilayer graphene is a solution for this problem, but it has a low band gap and therefore is not suitable for transistors.
Graphene is a honeycomb-like structure of carbon atoms that enables electrons to move quickly across its surface. Unlike copper, graphene electrons move at nearly the speed of light. This means that graphene electronics could charge within seconds or minutes, compared to hours with copper. And while it is difficult to predict exactly when graphene will replace semiconductor chips, it is clear that the technology has the potential to transform the world of electronics.
The future of computer technology depends on the use of new materials and processes. Graphene and nanomagnets are leading contenders. Graphene may not replace silicon chips for several decades, but nanomagnetic computing is the most promising replacement for silicon-based computer chips. And while quantum computing is still in the early stages, it may be decades away. However, it's still an exciting prospect that will allow researchers to build computer systems that do not need transistors.
Despite its popularity, it hasn't been easy to produce the new materials necessary for semiconductor manufacturing. Fortunately, a number of big companies are working to develop these new materials. However, it's still early days for these technologies and there are many problems to solve before the technology is ready to go commercially. And it may take years before the market matures enough to allow them to replace silicon. The key is to find a way to make them affordable and produce them in large quantities.
Silicon carbide
In addition to having superior energy efficiency, silicon carbide will also be cheaper. Unlike silicon, which requires days to grow a single crystal, silicon carbide requires less time. It can also be produced in 200-mm wafers, which will make it the standard size in the silicon industry. These features will make silicon carbide the next generation semiconductor. It will also provide significant benefits for the electronics industry. The technology will help silicon-based companies replace the high-cost silicon chips with more efficient and environmentally friendly alternatives.
Although silicon will still dominate the half-trillion dollar semiconductor industry, it may eventually be replaced by silicon carbide. Its specialized nature will allow it to resist higher charges than silicon. This will improve battery life and reduce energy losses from motors. Silicon carbide will also be used in power electronics, which will make them more efficient than their silicon counterparts. Silicon carbide's potential in automotive applications could be bigger than its potential in the semiconductor industry. Moreover, it could be used in solar and wind power systems and electric trains.
In electric cars, silicon carbide could replace the conventional silicon chips. According to a study conducted by the Biophysical Economics Institute and Wolfspeed, Inc., silicon carbide can provide a 13-to-1 energy savings. It can also significantly reduce the amount of battery required to power an electric vehicle. Silicon carbide's high energy efficiency allows for better range. The cost of electric cars may increase by $200, but the energy savings will be substantial.
Aside from EVs, silicon carbide is expected to take over the automotive industry in the near future. In addition to automotive, Silicon carbide is also widely used in clean-energy devices, such as HVAC and electric vehicles. Moreover, it is used in industrial applications, such as the production of a large number of electronic devices, which are run continuously. Silicon carbide can also make electronics more efficient. However, the future of semiconductors is unclear, and many companies are trying to position themselves as competitors to silicon carbide.
While silicon is still the most popular semiconductor material, it is beginning to show its limitations in high-power applications. A new material, SiC, is a possible replacement. SiC is a compound of silicon and carbon and belongs to the wide bandgap family of semiconductor materials. It has good chemical, thermal, and mechanical properties. Its low thermal resistance and strength make it an attractive choice for high-power devices.
Gallium nitride
The semiconductor technology of the future is a new material called gallium nitride, which is replacing silicon in many applications. It is used in power electronics such as 48 V DC/DC converters, which are used in computers and data centers. Gallium nitride-based solutions are also used in high-volume automotive applications. In the coming years, the material will be used in many products, including smartphones and laptops.
The main benefit of gallium nitride over silicon is its higher thermal stability. This means that it can sustain higher temperatures and more load without deteriorating. This makes gallium nitride the perfect future-proof semiconductor material. Its smaller size and high-frequency performance will make it easier to produce power products, such as GaN MOSFETs. But the real question is: how will it replace semiconductor chips?
Silicon has been a mainstay of the electronics industry for nearly 60 years, but it has reached its limits in some applications. In order to continue making faster circuits, research scientists have been searching for alternatives to silicon. Gallium nitride, which is more conductive than silicon, is a promising alternative. It has many advantages, including faster switching speed, smaller size, lower cost and increased efficiency.
One of the main benefits of gallium nitride is its ability to reduce power consumption. The technology has the potential to reduce power consumption by as much as 40%. This is important because as data traffic increases exponentially, it will require more bandwidth. By reducing power consumption, gallium nitride chips will dramatically increase the efficiency of data centers. These benefits will be realized through significant energy savings and environmental benefits.
The new material is much cheaper than silicon and is more efficient as a power converter. The energy conversion time of the chip can be cut in half. The company that invented gallium nitride claims that the new material will be used in electronics, including electric vehicles. Silicon-based chips are made by billions of units each year. This massive global infrastructure has made them relatively inexpensive. However, silicon-carbide wafers are still a difficult and expensive material to produce.
Molybdenum disulfide
Researchers are developing two-dimensional materials that could replace silicon in future computer chips and flexible electronics. These materials are called molybdenum disulfide, and they have been studied by many researchers across the world. The problem with silicon is that it has a very large electrical resistance, which limits the flow of current from one metal contact to another. Molybdenum disulfide can reduce that electrical resistance by up to a hundred times.
One advantage of molybdenum disulfide transistors is their small size. They can operate at a faster speed than silicon transistors. Researchers believe that these devices will be the future of semiconductor technology. The material's high ballistic conduction ratio is important for high-speed devices. It can also help improve the efficiency of transistors. Ballistic conduction is a key factor in enhancing transistors.
While Silicon remains the primary material for semiconductor chips, there are numerous promising materials that may replace it in the future. Molybdenum disulfide is a promising candidate for future electronic circuits. The material has several interesting properties, including an ultrathin channel thickness. This enables improved electrostatic gate control, reduces short-channel effects, and results in better geometric scaling behavior and less power consumption.
The material can be used as a substrate for different film surfaces. It is also used for thin-film solar energy and other coating industries. Eventually, molybdenum disulfide could replace silicon and graphene in semiconductors. This material is used for many other purposes, from machinery lubrication to new energy. The government has declared molybdenum a strategic metal.
Researchers hope to use the two-dimensional form of molybdenum disulfide to create LEDs and self-reporting sensors. These new materials could be used to replace the need for semiconductors altogether. The possibilities for using molybdenum disulfide in electronics are endless, and the researchers are excited about its future. This new material is expected to be widely used in the near future.
The researchers have a long way to go to create a 3D chip. However, their research at Stanford and MIT is proof of the progress that is being made in this field. Although a 3D chip has yet to be manufactured, the technology behind it can be used in flexible devices. This makes it a viable alternative to silicon for computer chips. It is also far cheaper than the current silicon devices.
Video: Goodbye Silicon!
Does Silicon React Electrovalently Or Covalently?
In chemistry, a bond is a chemical bond between two atoms or molecules that have a similar electron ![]() configuration. In general, silicon is not able to form more than four bonds with any other atom. Carbon, for instance, can form giant structures by covalently binding its atoms to one another. Covalent compounds can also form crystal lattices - complex structures made up of repeated units.
configuration. In general, silicon is not able to form more than four bonds with any other atom. Carbon, for instance, can form giant structures by covalently binding its atoms to one another. Covalent compounds can also form crystal lattices - complex structures made up of repeated units.
Silicium
An element's atomic number, its relative atomic mass, and its electron distribution will determine whether it reacts in an electrovalent or covalent way. The more positively charged an element is, the stronger its electrovalent bond will be. This is the case with the element silicon. Its atomic number is 14 and its relative atomic mass is 28.
Silicon is abundant and largely stable. It has four valence electrons and is found in many crystalline forms. Minerals that contain silicon include feldspar, micas, olivines, and pyroxene. It also occurs in water. In addition to crystalline silicon, this element is also found in brown amorphous form, which can be seen in dirty beach sand.
Crystals of silicium can be either electrovalent or covalent, which is based on the chemical bonds between the elements. Covalently bonded minerals are highly stable and exhibit a high melting point. Crystals of these minerals tend to show less symmetry than their ionic counterparts.
In a covalent way, silicium reacts more efficiently with its neighbors. This is due to a process called bond reconstruction. This process reduces the core energy of the dislocations, which causes the dangling bonds to reform with others to maintain their coordination. Electronic paramagnetic measurements of deformed silicon crystals indicate that most sites along the dislocation core are reconstructed.
Tectosilicates are silica compounds that have the properties of covalent solids. These materials have many uses in modern industry. Silicon and carbon-hydrogen compounds are the backbone of the living world. Their chemical versatility is a common theme, although the two substances are not intimately related in biology.
Ionic or electrovalent compounds
Ionic or electrovalent compounds of silicon are substances that have a positive or negative charge. When dissolved or melted, these materials lose their solid state and dissolve in water. This causes the crystal structure to be broken and the ions become free to move around, which allows them to conduct electricity. Ionic compounds are soluble in aqueous solutions and in water, and are very difficult to dissolve in other solvents.
Electron transfer is the primary mechanism of forming an ionic or electrovalent bond. This process results in one atom losing its electrons and one atom gaining an electron. The atom that loses its electron becomes positively charged, while the one that takes it is negatively charged. The two atoms then form an electrovalent bond.
The electron-exchange reaction is one of the most important parts of electrocatalysis. The presence of ionic bonds between two elements can make silicon an ideal material for catalysis. Silicon dioxide, for example, can act as a catalyst for chemical reactions. It has the ability to transfer electrons between molecules and is a valuable resource for electronics and the biomedical industry.
Silicon dioxide is a common compound found in nature. It is found in rocks and sand. It can also be extracted through mining. The process produces amorphous silicon dioxide. Silicon and oxygen have different electronegativities. Oxygen attracts shared electrons more than silicon, so it does not act as an ionic compound.
A pure ionic compound has a spherical lattice of ions. The electrons of negative ions can be polarized by small positive ions. This leads to partial covalency. A partial covalent compound is formed when the positive ions have a charge higher than the negative ones.
A good example of an electrovalent bond is a salt. It contains sodium and chloride. The sodium ion is positive, while sodium chloride is negative.
Its bonding versatility
Silicium's bonding versatility is a major advantage in a number of chemical processes. Its ability to form hydrogen bonds, which are similar to those found in other organic molecules, speeds up chemical reactions. Additionally, it can form covalent bonds with other molecules. In addition, selenium's bonding versatility makes it a very useful chemical activator.
The bonding versatility of silicon is largely due to the siloxane linkage, which allows the material to adopt a number of different forms. This diversity is achieved by using organic groups to control siloxane structures and modify their properties. Such studies are useful in enhancing the understanding of the chemical bonds between molecules.
Its valence number
Silicon is a nonmetal and like carbon has a valence number of four. This means that it can only form four bonds. Silicon is not stable when forming double bonds and does not exist in the graphite structure. When it forms a compound, four silicon atoms bond with one another, forming a covalent bond.
The chemical reaction occurs because the oppositely charged ions attract each other. Silicon, for example, forms a covalent halide and an acidic oxide. This is because silicon has an electron configuration of two, eight, and four. It needs four more electrons to achieve an inert gas configuration. In addition, it forms a covalent bond when it reacts with another metal, such as oxygen.
The elements in a periodic table are grouped according to their valence number. Period 1 elements have one electron, period 2 elements have two, and period 3 elements have three. In addition, elements within the same period have similar valence electron numbers.
Alkali metals are light metals with a low valence number and react with cold water to release hydrogen gas. These metals form ionic or electrovalent bonds and are exceptional conductors of electricity. They are produced through the electrolysis of hydroxides and chlorides. They should be stored in a sealed, inert atmosphere, under oil.
Unlike ionic bonding, which is characterized by its high melting and boiling points, covalent bonds are directional and highly stable. In addition, covalently bonded minerals generally have a lower degree of symmetry than their ionic counterparts.
Concept of Valency
Why is Silicon Used to Make Most Microprocessors?
Silicon is the second most abundant element on earth after oxygen. It's also very small. It is a ![]() semiconductor and can be found in the purest form in sand. Silicon is a semiconductor, which means that it can store information in a very small space.
semiconductor and can be found in the purest form in sand. Silicon is a semiconductor, which means that it can store information in a very small space.
Purest silicon is found in sand
Silicon is a common element found in the earth's crust. Its main form is oxide or silicate, and sand is a common source. But silicon is rarely found in nature in its pure form, which is needed for electronic applications. In order to achieve this, silicon must contain less than one non-silicon atom per billion. Silicon is often purified through different processes. Some methods are simple and can be carried out at home, while others require high temperatures.
Silicon is the second-most abundant element in the Earth's crust, accounting for about 25.7% of the planet's mass. It is found in sand and clay, and is a constituent of rock crystals, feldspar, and asbestos. Silicon is also found in water and in certain animals and plants.
Silicon has been compared to diamond, and it shares many of the same chemical properties. However, the chemical structure and composition of the two elements are quite different. While diamond has a crystalline structure, silicon is found in a brown powdery form called amorphous. This form is much more reactive than crystalline silicon.
Silicon is also used for making ceramics and refractory materials. High-purity silicon is used in the production of semiconductor devices. For this reason, silicon must be even purer than metallurgical grade silicon. Fortunately, there are a number of methods to produce high-purity silicon.
It's the second most abundant element on earth after oxygen
Silicon is a nonmetallic chemical element found in large quantities in the Earth's crust. It's the second most abundant element after oxygen and makes up nearly one-fourth of the planet's crust. Because silicon is so abundant, it's not unusual to find silicon in rock formations. In fact, silicon makes up 27.7% of the Earth's crust and is the second most abundant element after oxygen.
Silicon is a great choice for semiconductors because of its inherent insulating properties. When added to other elements, silicon transforms into metal-oxide-silicon (Glass). This material is incredibly efficient in insulating circuits, saving manufacturers time and money. It's also more abundant than most other semiconductors, making production much more cost-effective and significant. Silicon manufacturers don't need to worry about running out of raw materials, which makes the manufacturing process faster and more efficient.
Silicon was first isolated in 1824 by Swedish chemist Jons Jacob Berzelius using a technique similar to Davy's. Berzelius added a compound known as K2SiF6 to a molten metal. This combination produced a new element, and the name was soon suggested by Scottish chemist Thomas Thomson.
Silicon is also the second most abundant element on earth after oxygen, making it an excellent choice for microprocessor production. It's also important to note that silicon is naturally found in sand and rocks. However, its extraction is energy-intensive. A kilogram of high-grade silicon requires between 1000 and 1500 megajoules of primary energy to produce.
It's a semiconductor
Silicon is a common element and is found in abundance on Earth. It makes up over 25% of earth's crust by weight and is found in many forms including oxides and silicates. It is also found in metals and is produced through the reaction between silicon dioxide and carbon materials such as wood chips. The raw materials used to create silicon wafers come from many sources around the world, but China is believed to be the largest supplier.
Silicon is found in nature, and it is relatively inexpensive, making it an attractive material for electronics. Moreover, it is a near-perfect semiconductor, making it a good material for making computer chips. However, the disadvantage of silicon is that it is incredibly inefficient at converting light into an electrical signal and vice versa. As such, silicon semiconductors have historically been used in applications where they were connected to metal wires.
Silicon is a semiconductor used to make most transistors and microprocessors. Semiconductors have a wide range of properties and can be classified as N-type or P-type. N-type semiconductors have negatively charged electrons while P-type semiconductors contain electron deficiencies. Semiconductors can be made from several materials, but silicon is the most popular.
Silicon is also used to make many other electronic devices. These include LED lights and solar panels. It is the second most abundant element in the earth, and is found in most soil and rocks.
It's small
Silicon is one of the most commonly used materials in computer chips. Its unique properties make it the material of choice for most electronic chip components. Unlike many other metals, silicon is a semiconductive metalloid, meaning that it can allow current to flow between two opposite sides. The conductive properties of silicon are dependent on impurities. The presence of impurities in silicon makes it easier for transistors to switch from insulator to conductor when voltage is introduced.
Silicon is the second most abundant element in the world, making up more than 25% of the earth's crust. It occurs naturally as a mineral or in compounds called oxides or silicates. It is also produced from the reactions between carbon materials, such as wood chips, and silicon dioxide. While several countries around the world make silicon wafers, China is the world's leading producer.
Silicon is also more abundant on Earth than carbon. This makes it possible for life to develop on silicon. However, it is not clear whether silicon-based life will be as diverse as carbon-based life. It may require a wider range of reaction-driven enzymes to thrive.
The main component of a microprocessor is silicon. While this material is a common element, it is extremely difficult to use in pure form. A semiconductor cannot be used in a pure state, but can be converted to a more usable state by removing oxygen from it.
It's fast
Silicon is a common element found in the Earth's crust, and is an excellent semiconductor material. The element is also widely available and relatively cheap to produce. Since silicon has a wide temperature range and can be doped easily with certain chemicals, it's a good choice for making computer chips.
Silicon is an element that can be found in most building materials, and is also used to make most digital computer processors. It is the basis of modern electronic devices, including smart phones and air microwave ovens. It is a semiconductor, which means that it can conduct electricity under some conditions but act as an insulator under others. In addition, silicon can be modified in order to modify its electrical properties.
Silicon has an extremely low resistance and can be used to make transistors. However, the element is rarely used in its pure state. This is because copper lets electrons pass through too easily, so too many transistors would light up simultaneously, which prevents the CPU from processing instructions.
Silicon crystals are produced in 300-mm diameter cylinders. However, researchers are quickly approaching the 450-mm threshold. This will allow for increased speeds and lower production costs. The downside to making large silicon crystals is that they may become less plentiful and harder to work with.
It's cheap
Silicon is a very common material for microprocessors, and it is cheap to produce. It is an insulator by nature, but it can be made into a conductor when it is exposed to oxygen. This makes silicon an ideal material for computer chips. This property is also a key factor for making silicon chips cheap.
Silicon is one of the most abundant elements on earth, second only to oxygen. However, it's not a pure material, and is rarely used in its pure form. The process for making silicon chips begins with mining silicon dioxide, a natural substance that can be found in sand. Once extracted, the silicon is melted into a cylinder, or ingot. This ingot is then sliced into thin wafers. The thinness of these wafers is measured in nanometers, which is a millionth of a millimeter.
A silicon chip is a single piece of silicon that contains thousands of transistors. Transistors act like miniature switches, turning electrical current on or off. By adding and removing material, silicon becomes a pattern of tiny switches. Silicon is cheap, making it a great choice for microprocessor chips.
While silicon chips are cheap and easily manufactured, they don't have the properties of the best material. Silicon isn't naturally thin, flexible, or conformable. The process uses chemicals that allow it to be etched to a finer scale.
Video: Silicon Microprocessor
How Are Silicon Wafers Measured to Be a Centimeter Long?
Silicon wafers are a common material used in semiconductors and other electronic components. ![]() Depending on their size, they can be measured in various ways. These methods include Four-point probe method, Orientation flats, and Reference notch. Read on to learn more.
Depending on their size, they can be measured in various ways. These methods include Four-point probe method, Orientation flats, and Reference notch. Read on to learn more.
Four-point probe method
Four-point probe measurements are a key part of electrical characterisation of materials. These measurements are often used to measure the thickness of silicon wafers. The four-point probe head has rounded tips to avoid piercing the thin film. Its constant contact force of 60 grams enables it to provide a good electrical contact.
The four-point probe method is usually the preferred method and has several advantages. However, this method is only useful for measuring thin film materials. The thickness of the silicon wafer cannot be accurately measured using this method. Furthermore, it requires the use of a multi-point electrode.
The measurement of non-metallic materials is crucial to the study of electronic surfaces. However, it is difficult to achieve a stable measurement due to the presence of various types of contact resistance. In the case of hard carbon fiber paper, for example, the surface is not uniform. It also undergoes oxidation, which damages the sp2 conjugation of carbon, resulting in lower conductivity. Furthermore, two-point probe measurements using uncoated stainless steel needles are noisy and result in an extreme bias in resistance.
Four-point probe measurements are based on the measurement of sheet and surface resistance. This technique measures sheet resistance in ohms per square. The four-point probe method is suitable for busy labs with limited shelf space. A PC software program is used to perform the measurements. The computer can also perform calculations relating to resistivity, sheet resistance and conductivity. The computer software can automatically correct for contact resistance.
When measuring a silicon wafer, it is necessary to know how thick the layer is. This measurement is not accurate if the layer is more than half of the measurement area. To achieve accurate measurements, you must have an electrically insulating substrate.
Orientation flats
The atomic structure of silicon is triangular, and therefore it is possible to measure the length of a silicon wafer to the nearest centimeter. In order to do so, the material must be heat-treated to 780 degrees Celsius for 3 h, and then cooled to a temperature of 1000 degrees Celsius for 16 h. Once the material is cooled, the BMD density is measured every 2 mm along the wafer's radii with a SIRM at a depth of 50 mm.
To measure the length of silicon wafers, the surface of the silicon wafer must be flat and rotationally symmetric. This requires a backside coating of Polysilicon, which is designed to draw defects away from the front side. The radial positions of the two flats must be consistent and the primary flat must be 0.5 degrees cw from the secondary flat.
Wafers can be manufactured with a variety of edge profiles. For example, a 150-millimeter silicon wafer may have only one flat, while a 200-millimeter silicon wafer may have a notch instead. The reason for this is to conserve the surface area for devices.
The center region of a silicon wafer is called the COP disk. This is the region on the wafer's surface that contains agglomerated crystal lattice vacancies. These agglomerates have a density of more than six times that of the wafer's length.
The v/G is a measure of the vacancy concentration. The central part of the wafer has the highest vacancy concentration. The v/G ratio is an important parameter in silicon wafers. If the v/G is lower than this, then oxygen precipitation will occur at lower temperatures. This increases the yield of type S2 wafers, which lie completely inside the vacancy-rich region II.
The most popular method is called the four-point probe method. This method is good for thin films, but has limitations. For example, it is difficult to measure the thickness of a silicon wafer using the four-point method.
Reference notch
A reference notch on silicon wafers is a hole located on the surface of the silicon wafer. It helps in determining the orientation of the silicon wafer. For instance, if the wafer is 200 mm in diameter, a reference notch will be located at the 200 mm mark. Using this information, one can determine the orientation of the silicon wafer in a chip design.
There are two types of reference notches. The first is a small depression in the surface of the semiconductor wafer, and the second is a larger depression. Both of these are used to define the orientation of the crystal. These notches serve to suppress slip lines, which are often generated by thermal processing.
The second type is defined as an extension of the first notch. The first notch is V-shaped, while the second notch is semicircular. The notch is defined by a line extending from vertices 25 and 26. In this way, it can be easily identified by the orientation of the crystal.
The reference notch on silicon wafers allows users to see crystal defects in a more accurate manner. In addition, it prevents the wafer from becoming unaligned. In other words, it shows which side of the silicon is aligned with which side of the semiconductor wafer.
The second reference notch on silicon wafers is located on the outer periphery of the semiconductor wafer. It intersects with the outer periphery at a prescribed angle. The first and second notches are placed adjacent to each other. In this way, the semiconductor wafer can be easily differentiated.
During the process of manufacturing silicon wafers, a reference notch is created on the silicon wafer's surface. This notch helps manufacturers identify the crystal orientation. Using the reference notch, manufacturers can easily identify which side of the silicon wafer has a higher conductive area. This allows manufacturers to make more efficient products.
Another way to identify the semiconductor-grade silicon is through the Czochralski process. In this process, silicon is melted in a quartz crucible at 1425degC. Boron and phosphorus are added as dopant impurities. The result is a semiconductor that is p or n-type.
Resistivity measurement
The simplest method to perform a resistivity measurement on silicon wafers involves a four-point probe technique. This method is useful for determining the thickness of the silicon wafer. The measurement should be performed at different levels of resistance. This test can be done on a centimeter-length silicon wafer.
The four-point probe array measures the variation in resistivity between the center of the wafer and selected outer regions. However, the amount of information that can be obtained depends on the sampling plan. If the axial and azimuthal variation along the length of the crystal are not negligible, the variations measured in this manner may not be correct.
The results of the resistivity measurement on a centimeter-long silicon wafer are expressed as changes in resistance between two adjacent measurement sites. These results should be corrected for the specimen temperature. In addition, values are given in SI units and in parentheses as information only. Users should establish appropriate safety practices and identify any regulatory restrictions prior to performing any measurements using this standard.
The voltmeter is another tool that is used for measuring resistivity on silicon wafers. A voltmeter with high accuracy and precision is crucial. The accuracy of the measurement is essential for determining the quality of the semiconductor. A voltmeter with a microvolt reading is useful for measuring very thin films and for thicker films.
The method presented in this paper demonstrates 0.6% 1-sigma repeatability over a five-day period. The accuracy of the measurement is due to the precise control of the surface charge in the wafer with the integrated preconditioning equipment. Moreover, the measured resistivity results show a stable state of inversion.
The minimum resistivity gradient is dependent on a number of parameters. The smaller the number of electron-hole pairs, the lower the minimum resistivity gradient will be. Higher-resolution resistivity measurements will require a longer carrier lifetime and a higher radiation intensity. However, the spatial resolution will be poorer. The minimum resistivity gradient is typically 1.0 O * cm/cm.
The resistance of a silicon wafer is a function of its volume. The volume resistivity is the ratio of the potential gradient and
Why is Silicon Used As a Conductor in Making Transistors and Chips?
Basically, silicon is used as a conductor in making transistors and chips because it has a high electrical conductivity, and there are ways to change this electrical conductivity in a chip. For example, if you use photolithography, you can change the conductivity of parts of the chip. Another way to change the conductivity of parts of the chip is to use other materials, such as boron or Phosphorus. These materials are similar to silicon in terms of their appearance, but they are able to grab electrons from the silicon lattice.
Phosphorus sheds electrons to fit in with silicon lattice
Adding phosphorus to silicon can have a huge effect on silicon's conductive behavior. For instance, the extra electron it adds to the lattice may jump into the conduction band, resulting in a significantly higher conductivity. However, the extra electron is not stable and may move around the crystal.
For example, the extra electron can be transferred to the nucleus. This can cause a phenomenon known as the quantized electron pump. It is a charge pumping process that produces an electron that has an overall negative charge.
Phosphorus is also a doping material. It can be used to increase the concentration of singly negatively charged native defects in silicon. In a recent study, researchers have used this type of doping to simultaneously investigate phosphorus' self-diffusion and Si's self-diffusion. This allows a qualitative description of how the two species interact.
Phosphorus is an N-type doping material that forms four covalent bonds with adjacent silicon atoms. The resulting molecule has five valence electrons. However, only four of these electrons are in the valence band.
The extra electron can be transferred to the nucleus of the molecule, where it can be read out after 80 ms. The energy of the atom containing the extra electron is closer to that of the valence band. However, it is not as energy intensive as the energy of the filled valence band.
Adding phosphorus to silicon can also increase the amount of electrons that are pumped into the silicon lattice, which is a good thing. The extra electron can move from one place to another in the crystal, creating a positive charge. This is called the Kohn-Sham eigenvalue.
Boron atoms grab electrons out of the silicon lattice
During the discovery of boron, scientists searched for the substance in a variety of mineral deposits around the world. Finally, they found it in Himalayan pink rock salt.
When boron atoms are added to the silicon lattice, they grab electrons that have been pushed out of the lattice. This is called doping. Doping can be achieved by diffusion or by ionic implantation. Using ionic implantation, dopants can be inserted deep inside the crystal lattice. Doping is usually used in the production of transistors and chips.
The boron atom has five protons and five electrons. The outermost shell holds three of the electrons, while the remaining two are held in the second shell. The boron atom is able to form bonds with three silicon atoms.
The first shell holds two electrons. The boron atom has the advantage of being able to form a valence bond with four of the neighboring silicon atoms. These bonds create a net positive charge.
The valence band holds the highest energy electrons. This band is sometimes called the valence shell. A valence bond is when two atoms donate electrons to form a bond. This type of bond is also called a sharing bond. The atoms simultaneously borrow an electron from the other atom, but remain electrically neutral.
The real answer to the semiconductor question is usually given with the physics equations, or with names like Fermi's Law. It is also important to note that the real answer is usually provided without any math formulas.
One of the most important properties of a semiconductor is the transfer of electrical charge. A semiconductor's band gap, or the number of electrons in the conduction band, determines the colour of light that is produced by a light beam. A light beam's energy level must be greater than the band gap for the light to be visible.
Germanium is a chemical element that is similar in appearance to silicon
During the early 1950's, Germanium was the dominant semiconductor material. It was used in a variety of applications. It was also used in the creation of the first crude integrated circuit. Robert Noyce hand-built the world's first integrated circuit on September 12, 1958.
In 1869, Dmitri Mendeleev predicted the existence of an element called germanium. He believed the element would be found in the gaps of the periodic table that remained blank. He called it ekasilicon, and estimated the element's atomic weight to be 70.
In 1886, Clemens Winkler was the first to discover germanium. He discovered the element in a mineral called argyrodite, and named it after his home country, Germany.
After being isolated, it was discovered that germanium has similar chemical properties to silicon. It is used as a semiconductor and is a common component in integrated circuits. It has a metallic luster and is hard and brittle.
Although it is not considered an essential element for living organisms, it does have important applications in the electronics industry. It is used in semiconductors and lasers. It is also used as a doping agent for silicon.
In addition to being a semiconductor, it is also a good insulator. It is found in sand and other minerals, as well as in the Earth's crust. It has been detected in certain types of meteorites.
Its crystalline form is a gray-white metalloid. It has a luster similar to diamond. It is also used in photodetectors and lasers. It can be extracted from copper ores, as well as silver and lead ores.
It is often extracted as a byproduct of zinc refining. Its production is largely led by China.
Photolithography can alter the electrical conductivity of parts of the chip
During the fabrication of semiconductor devices such as transistors and chips, a photosensitive coating material is used to define the circuit patterns on the wafer's surface. This coating material becomes soluble when exposed to ultraviolet light. In turn, these wavelengths of light enable the material to be etched off of the wafer's surface.
Silicon-based materials dominate the semiconductor industry. The properties of these materials depend on the presence of impurities. Silicon, for example, has a low electrical conductivity, and this is due to small amounts of impurities. However, a chemical called boron can be added to the silicon lattice to generate a positive charge. This increases the electrical conductivity of the material.
A junction diode is a semiconductor device that emits light when a current passes through it. It is used in early radios as a signal detector. The device converts alternating current to direct current.
The junction of n-type and p-type semiconductors creates an electronic barrier. This barrier protects the semiconductor from atmospheric conditions, corrosion, and other external stresses. It also improves the life of the semiconductor.
Silicon wafers have bulk resistivity between 1 and 10 O-cm. Their thickness is approximately 325um. A thin layer of silicon dioxide is then thermally grown on top of the wafer to form a hard mask for etching the silicon.
Doping is a chemical process that changes the conductivity of a semiconductor. It involves adding small amounts of doping atoms such as phosphorous or boron to the silicon lattice. The doping atoms promote electrons to a higher energy level, and thus, a semiconductor becomes a good conductor.
The valence band is a region in a semiconductor where electrons are in bonding states. The band gap is smaller here, making it easier for electrons to jump to the conduction band.
GaN could replace silicon as the preferred semiconductor for the power electronics industry
Currently, silicon is the dominant semiconductor. But it's not the only option. A new material called Gallium Nitride (GaN) could replace silicon as the preferred semiconductor for the power electronics industry.
The enticing properties of GaN are attracting manufacturers. For example, a GaN power IC chip can save up to 80% in manufacturing costs. It can also operate reliably at higher temperatures. The chip can also be three times smaller than silicon-based designs, enabling faster charging of electric vehicles.
The semiconductor can be used in a variety of power switching applications. It's particularly well-suited for high-power transistors. It can sustain higher voltages than silicon, which results in smaller devices and lower energy losses.
Gallium Nitride can also be used in solar inverters. It's a common component in light-emitting diodes (LEDs), which are widely used in LED lighting. GaN is also used in sensor technology. In fact, GaN has been used in LED production since the 1990s.
As technology continues to evolve, GaN's capabilities are expected to grow as the backbone of power switching technology. This will help meet the growing energy demand and reduce carbon emissions.
The power electronics industry is expected to see a huge boost in GaN adoption in the next decade. One of the biggest developments is mobile fast charging. GaN power ICs can charge electric vehicles three times faster than silicon-based chips. This will increase consumer acceptance of electric vehicles.
The world's largest chip foundry, TSMC, recently invested in 16 specialist pieces of machinery to meet the demand for GaN chips. This investment is part of a multi-billion-dollar electrification opportunity for the company.
Silicon FAQs
Question: Does Silicon Conduct Electricity?
Answer: Yes, silicon is a conductor of electricity. It is a semiconductor, which means that it has the ability to conduct electricity under certain conditions, but not as well as a metal. The electrical conductivity of silicon can be modified by introducing impurities into the material, a process known as doping. Silicon that has been doped with impurities such as phosphorus or boron is called n-type silicon, and it has an excess of electrons that make it more conductive. Silicon that has been doped with impurities such as aluminum or gallium is called p-type silicon, and it has a deficiency of electrons, known as "holes," which allows it to conduct electricity.
Silicon is widely used in the electronics industry to make transistors, which are the basic building blocks of electronic devices such as computers, smartphones, and televisions. It is also used to make solar cells, which convert sunlight into electricity, and is an important component in many other electronic devices.
What are Five Methods Used to Increase Silicon Wafer Yields
-
Silicon Surface preparation: Proper surface preparation, including cleaning and polishing, is crucial for improving silicon wafer yields. Clean surfaces minimize the risk of defects and improve the quality of the final product.
-
Quality control: Implementing strict quality control measures and inspecting wafers at various stages of processing can help identify and prevent defects before they become widespread.
-
Improved process control: Maintaining tight control over the processing parameters and conditions helps to ensure that the wafers are produced consistently and with minimal defects.
-
Advanced manufacturing techniques: Adopting advanced manufacturing techniques, such as chemical-mechanical polishing, can help improve the yield of silicon wafers.
-
Equipment maintenance: Regular maintenance and calibration of the equipment used in the manufacturing process helps to ensure that the equipment is operating correctly and producing wafers with minimal defects.
By implementing these methods, manufacturers can improve the yield of silicon wafers, reducing waste and improving the efficiency of the manufacturing process.
What is Silicon Absorption?
Silicon absorption refers to the ability of silicon to absorb certain wavelengths of light. Silicon is a semiconductor material commonly used in the production of solar cells. When silicon absorbs light, it can create free electrons and positively charged holes, which can be separated to generate an electrical current. The efficiency of a solar cell depends on its ability to absorb as much light as possible, which is why researchers continue to study ways to improve the silicon absorption process.
What is The Spectrum?
The spectrum refers to the range of wavelengths of electromagnetic radiation, which includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. Each type of electromagnetic radiation has a unique wavelength, frequency, and energy level, and the entire range of wavelengths is referred to as the electromagnetic spectrum. The visible spectrum, which is the range of wavelengths that the human eye can detect, includes the colors of the rainbow, from violet to red. Scientists and engineers use the spectrum to study and manipulate electromagnetic radiation for a variety of purposes, including communication, imaging, and energy production.
What is Absorption Coefficient of Silicon?
he absorption coefficient of silicon is a measure of how much of a specific wavelength of light is absorbed by silicon as it passes through the material. It is expressed in units of inverse length, typically in cm^-1. The absorption coefficient depends on the wavelength of the light and the physical properties of the silicon, such as its crystal structure and doping level. A higher absorption coefficient means that more light is absorbed by the material, while a lower absorption coefficient means that more light can pass through it. In the context of solar cells, a high absorption coefficient is desirable to maximize the amount of light that can be converted into electricity. Researchers study and manipulate the absorption coefficient of silicon to improve the efficiency of solar cells and other photonic devices.
